- Record: found
- Abstract: found
- Article: found
Computational analysis of naturally occurring resistance-associated substitutions in genes NS3, NS5A, and NS5B among 86 subtypes of hepatitis C virus worldwide
Read this article at
Abstract
Background and objective
Direct-acting antivirals (DAA) facing resistance continue to be used in some areas worldwide. Thus, identifying hepatitis C virus (HCV) genotypes/subtypes and loci with certain prevalent resistance-associated substitutions (RASs) deserves attention. We investigated the global and regional frequencies of naturally occurring RASs among all confirmed HCV subtypes (n=86) and explored co-occurring and mutually exclusive RAS pairs within and between genes NS3, NS5A, and NS5B.
Methods
A total of 213,908 HCV sequences available as of July 10, 2019 were retrieved from the NCBI nucleotide database. After curation, 17,312 NS3, 8,478 NS5A, and 25,991 NS5B sequence fragments from DAA-naïve patients were screened for RASs. MEGA 6.0 was used to translate aligned nucleotide sequences into amino acid sequences, and RAS pairs were identified by hypergeometric analysis.
Results
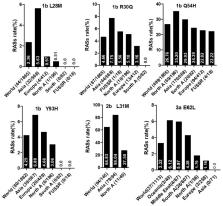
RAS prevalence varied significantly among HCV subtypes. For example, D168E, highly resistanct to all protease inhibitors except voxilaprevir, was nearly absent in all subtypes except in 43.48% of GT5a sequences. RASs in NS3 exhibiting significantly different global distribution included Q80K in GT1a with the highest frequency in North America (54.49%), followed by in Europe (22.66%), Asia (6.98%), Oceania (6.62%), and South America (1.03%). The prevalence of NS3 S122G in GT1b was highest in Asia (26.6%) and lowest in Europe (2.64%). NS5A L28M, R30Q, and Y93H in GT1b, L31M in GT2b, and NS5B C316N in GT1b was most prevalent in Asia. A150V in GT3a, associated with sofosbuvir treatment failure, was most prevalent in Asia (44.09%), followed by Europe (31.19%), Oceania (24.29%), and North America (19.05%). Multiple mutually exclusive or co-occurring RAS pairs were identified, including Q80K+R155K and R155K+D168G in GT1a and L159F+C316N and R30Q (NS5A)+C316N (NS5B) in GT1b.
Related collections
Most cited references49
- Record: found
- Abstract: not found
- Article: not found
EASL Recommendations on Treatment of Hepatitis C 2015.
- Record: found
- Abstract: found
- Article: not found
Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose.
- Record: found
- Abstract: found
- Article: not found