- Record: found
- Abstract: found
- Article: found
CXCL10 Produced by HPV-Positive Cervical Cancer Cells Stimulates Exosomal PDL1 Expression by Fibroblasts via CXCR3 and JAK-STAT Pathways
Read this article at
Abstract
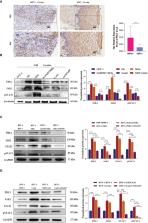
Persistent infection with human papillomavirus (HPV) and immune surveillance failure may be the initiating factors for the carcinogenesis of cervical squamous cell carcinoma (CSCC). HPV infection might affect the innate immune pathway of cervical epithelial cells that constitute the “microenvironment” for tumor cells. Programmed death-ligand 1 (PD-L1) has been reported to be an immunosuppressor that helps cancer cells escape the actions of T cells. In the present study, CXCL10 was substantially upregulated both in cervical tissues of HPV infected patients with cervical intraepithelial neoplasia (CIN) or CSCC, as well as in HPV16 E6/E7 transgenic murine cervix. The HPV-positive (HPV+) cervical cancer cell lines SiHa and Caski secreted increased levels of CXCL10 compared to human foreskin fibroblasts (HFF-1), and its receptor CXCR3 was overexpressed in HFF-1. After co-culture with SiHa or Caski, the JAK-STAT signaling pathway and exosomal PD-L1 expression were both upregulated in HFF-1. Recombinant human CXCL10 induced JAK-STAT and PD-L1, while the CXCL10-CXCR3 and JAK-STAT inhibitors AMG487 or ruxolitinib reduced the expression of PD-L1 in HFF-1 cells. Furthermore, the upregulated expression of PD-L1 was verified in HPV+ but not HPV-negative (HPV-) patients with cervical cancers by analysis of tissue microarray cores in 25 cervical lesion patients ( P < 0.05). The results indicate that HPV infection can induce cervical cancer cells to secrete CXCL10, which binds to CXCR3 in the surrounding fibroblast cells,leading to JAK-STAT pathway activation and the subsequent upregulated expression of exosomal PD-L1. These mechanisms may help HPV to escape immune response attack, leading to carcinogenesis.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Cancer immunoediting: from immunosurveillance to tumor escape.
- Record: found
- Abstract: found
- Article: not found
Toll-like receptors and their crosstalk with other innate receptors in infection and immunity.
- Record: found
- Abstract: found
- Article: not found
Roles for chemokines in liver disease.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.