- Record: found
- Abstract: found
- Article: found
Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy
Read this article at
Abstract
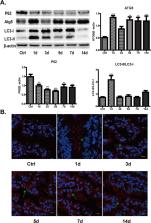
Tight junction barrier is critical to intestinal homeostasis. Applying antibiotics to treat infections is common in clinical practice, which may affect intestinal microbiota. Intestinal microbiota dysbiosis is involved in the occurrence of some gastrointestinal diseases. Therefore, this study was aimed to investigate the influence of antibiotics on intestinal tight junction barrier and the possible underlying mechanisms. Healthy adult female C57BL/6 mice were treated with a broad-spectrum antibiotic cocktail for 14 days. 16S rDNA Illumina sequencing and headspace gas chromatography-mass spectrometry (HS-GC/MS) were respectively used to analyze microbial community and to detect short-chain fatty acids (SCFAs) contents. In vivo intestinal paracellular permeability to fluorescein isothiocyanate-dextran (FITC-dextran) was measured. Protein expression was determined by immunoblotting. Immunofluoresence was applied to observe the distributions of ZO-1, LC3B and ASC. Antibiotics remarkably altered intestinal microbiota composition in healthy mice, accompanying reduced SCFAs’ concentrations. In addition, the intestinal tight junction barrier was disrupted by antibiotic treatment, as evidenced by increased intestinal paracellular permeability to FITC-dextran, decreased tight junction protein expressions, and disrupted ZO-1 morphology. Furthermore, NLRP3 inflammasome and autophagy were activated by antibiotic treatment. In conclusion, intestinal epithelial tight junction barrier dysfunction induced by antibiotics is associated with intestinal microbiota dysbiosis, activated NLRP3 inflammasome and autophagy in mice.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis.
- Record: found
- Abstract: found
- Article: not found
Antibiotic-Induced Changes in the Intestinal Microbiota and Disease.
- Record: found
- Abstract: found
- Article: not found