- Record: found
- Abstract: found
- Article: found
Identification of film-based formulations that move mRNA lipid nanoparticles out of the freezer
Read this article at
Abstract
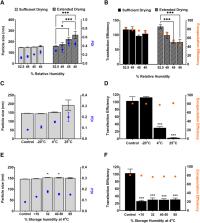
COVID-19 vaccines consisting of mRNA lipid nanoparticles (LNPs) encoding the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein antigen protected millions of people from severe disease; however, they must be stored frozen prior to use. The objective of this study was to evaluate the compatibility and stability of mRNA LNPs within a polymer-based film matrix. An optimized formulation of polymer base, glycerol, surfactants, and PEGylated lipid that prevents damage to the LNP due to physical changes during the film-forming process (osmotic stress, surface tension, spatial stress, and water loss) was identified. Surfactants added to LNP stock prior to mixing with other film components contributed to this effect. Formulations prepared at pH ≥ 8.5 extended transfection efficiency beyond 4 weeks at 4°C when combined with known nucleic acid stabilizers. mRNA LNPs were most stable in films when manufactured in an environment of ∼50% relative humidity. The optimized formulation offers 16-week stability at 4°C.
Graphical abstract
Abstract
Croyle and colleagues identify key factors in formulation composition, manufacturing, and storage conditions that offers 16week stability at 4°C for mRNA lipid nanoparticles (LNPs) within a film matrix. This exceeds current storage conditions of and global access to marketed mRNA LNP vaccines and other mRNA products.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Lipid nanoparticles for mRNA delivery
- Record: found
- Abstract: found
- Article: not found
mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability
- Record: found
- Abstract: found
- Article: not found
