- Record: found
- Abstract: found
- Article: not found
Activation of Disulfide Redox Switch in REDD1 Promotes Oxidative Stress Under Hyperglycemic Conditions
Read this article at
Abstract
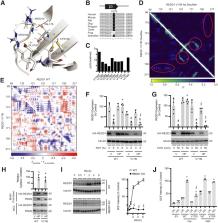
The stress response protein regulated in development and DNA damage response 1 (REDD1) has been implicated in visual deficits in patients with diabetes. The aim here was to investigate the mechanism responsible for the increase in retinal REDD1 protein content that is observed with diabetes. We found that REDD1 protein expression was increased in the retina of streptozotocin-induced diabetic mice in the absence of a change in REDD1 mRNA abundance or ribosome association. Oral antioxidant supplementation reduced retinal oxidative stress and suppressed REDD1 protein expression in the retina of diabetic mice. In human retinal Müller cell cultures, hyperglycemic conditions increased oxidative stress, enhanced REDD1 expression, and inhibited REDD1 degradation independently of the proteasome. Hyperglycemic conditions promoted a redox-sensitive cross-strand disulfide bond in REDD1 at C150/C157 that was required for reduced REDD1 degradation. Discrete molecular dynamics simulations of REDD1 structure revealed allosteric regulation of a degron upon formation of the disulfide bond that disrupted lysosomal proteolysis of REDD1. REDD1 acetylation at K129 was required for REDD1 recognition by the cytosolic chaperone HSC70 and degradation by chaperone-mediated autophagy. Disruption of REDD1 allostery upon C150/C157 disulfide bond formation prevented the suppressive effect of hyperglycemic conditions on REDD1 degradation and reduced oxidative stress in cells exposed to hyperglycemic conditions. The results reveal redox regulation of REDD1 and demonstrate the role of a REDD1 disulfide switch in development of oxidative stress.
Related collections
Most cited references51

- Record: found
- Abstract: not found
- Article: not found
