- Record: found
- Abstract: found
- Article: not found
Human disease models in drug development
Read this article at
Abstract
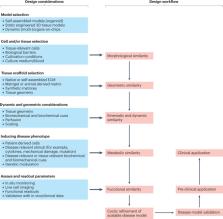
Biomedical research is undergoing a paradigm shift towards approaches centred on human disease models owing to the notoriously high failure rates of the current drug development process. Major drivers for this transition are the limitations of animal models, which, despite remaining the gold standard in basic and preclinical research, suffer from interspecies differences and poor prediction of human physiological and pathological conditions. To bridge this translational gap, bioengineered human disease models with high clinical mimicry are being developed. In this Review, we discuss preclinical and clinical studies that benefited from these models, focusing on organoids, bioengineered tissue models and organs-on-chips. Furthermore, we provide a high-level design framework to facilitate clinical translation and accelerate drug development using bioengineered human disease models.
Abstract
Owing to the high failure rates of the current drug development process, biomedical research is undergoing a paradigm shift towards approaches centred on human disease models. This Review critically discusses translationally relevant examples and defines key milestones for their widespread application.
Key points
-
Advances in bioengineering have yielded complex human disease models with high clinical biomimicry and predictability.
-
Human disease models can help unravel disease mechanisms, including for infectious and genetic diseases and cancer.
-
Using appropriate human disease models in the drug development process and clinical decision-making improves the rate of clinical translation, reduces costs and directly benefits patients.
-
Stringent model validation, regulatory and legal guidance, and scalable disease model production are key future milestones to facilitate their implementation in (pre-)clinical research.
Related collections
Most cited references191
- Record: found
- Abstract: found
- Article: not found
Sex differences in immune responses
- Record: found
- Abstract: found
- Article: not found
Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2
- Record: found
- Abstract: found
- Article: not found
