- Record: found
- Abstract: found
- Article: found
Genome analysis reveals insights into physiology and longevity of the Brandt’s bat Myotis brandtii
Read this article at
Abstract
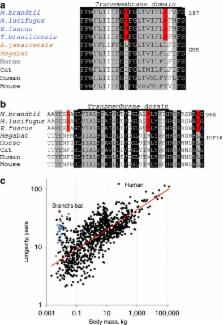
Bats account for one-fifth of mammalian species, are the only mammals with powered flight, and are among the few animals that echolocate. The insect-eating Brandt’s bat ( Myotis brandtii) is the longest-lived bat species known to date (lifespan exceeds 40 years) and, at 4–8 g adult body weight, is the most extreme mammal with regard to disparity between body mass and longevity. Here we report sequencing and analysis of the Brandt’s bat genome and transcriptome, which suggest adaptations consistent with echolocation and hibernation, as well as altered metabolism, reproduction and visual function. Unique sequence changes in growth hormone and insulin-like growth factor 1 receptors are also observed. The data suggest that an altered growth hormone/insulin-like growth factor 1 axis, which may be common to other long-lived bat species, together with adaptations such as hibernation and low reproductive rate, contribute to the exceptional lifespan of the Brandt’s bat.
Abstract
 Bats account for 20 per cent of all mammals, these are the only mammals with powered
flight, and are among the few animals that echolocate. Here, Seim
et al. sequence the genome of the long-lived (>40 years) Brandt’s bat,
Myotis brandtii and provide clues to its evolution, longevity and other traits.
Bats account for 20 per cent of all mammals, these are the only mammals with powered
flight, and are among the few animals that echolocate. Here, Seim
et al. sequence the genome of the long-lived (>40 years) Brandt’s bat,
Myotis brandtii and provide clues to its evolution, longevity and other traits.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
The evolution of maximum body size of terrestrial mammals.

- Record: found
- Abstract: found
- Article: found