- Record: found
- Abstract: found
- Article: found
Modulation of Host Immunity by Human Respiratory Syncytial Virus Virulence Factors: A Synergic Inhibition of Both Innate and Adaptive Immunity
Read this article at
Abstract
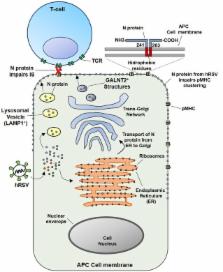
The Human Respiratory Syncytial Virus (hRSV) is a major cause of acute lower respiratory tract infections (ARTIs) and high rates of hospitalizations in children and in the elderly worldwide. Symptoms of hRSV infection include bronchiolitis and pneumonia. The lung pathology observed during hRSV infection is due in part to an exacerbated host immune response, characterized by immune cell infiltration to the lungs. HRSV is an enveloped virus, a member of the Pneumoviridae family, with a non-segmented genome and negative polarity-single RNA that contains 10 genes encoding for 11 proteins. These include the Fusion protein (F), the Glycoprotein (G), and the Small Hydrophobic (SH) protein, which are located on the virus surface. In addition, the Nucleoprotein (N), Phosphoprotein (P) large polymerase protein (L) part of the RNA-dependent RNA polymerase complex, the M2-1 protein as a transcription elongation factor, the M2-2 protein as a regulator of viral transcription and (M) protein all of which locate inside the virion. Apart from the structural proteins, the hRSV genome encodes for the non-structural 1 and 2 proteins (NS1 and NS2). HRSV has developed different strategies to evade the host immunity by means of the function of some of these proteins that work as virulence factors to improve the infection in the lung tissue. Also, hRSV NS-1 and NS-2 proteins have been shown to inhibit the activation of the type I interferon response. Furthermore, the hRSV nucleoprotein has been shown to inhibit the immunological synapsis between the dendritic cells and T cells during infection, resulting in an inefficient T cell activation. Here, we discuss the hRSV virulence factors and the host immunological features raised during infection with this virus.
Related collections
Most cited references84
- Record: found
- Abstract: found
- Article: not found
Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus.
- Record: found
- Abstract: not found
- Article: not found
Respiratory syncytial virus and parainfluenza virus.
- Record: found
- Abstract: found
- Article: not found