- Record: found
- Abstract: found
- Article: found
Total Flavonoids from Clinopodium chinense (Benth.) O. Ktze Protect against Doxorubicin-Induced Cardiotoxicity In Vitro and In Vivo
Read this article at
Abstract
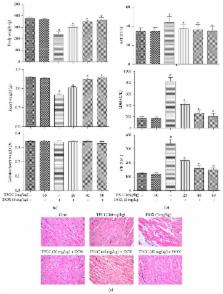
Doxorubicin has cardiotoxic effects that limit its clinical benefit in cancer patients. This study aims to investigate the protective effects of the total flavonoids from Clinopodium chinense (Benth.) O. Ktze (TFCC) against doxorubicin- (DOX-) induced cardiotoxicity. Male rats were intraperitoneally injected with a single dose of DOX (3 mg/kg) every 2 days for three injections. Heart samples were collected 2 weeks after the last DOX dose and then analyzed. DOX delayed body and heart growth and caused cardiac tissue injury, oxidative stress, apoptotic damage, mitochondrial dysfunction, and Bcl-2 expression disturbance. Similar experiments in H9C2 cardiomyocytes showed that doxorubicin reduced cell viability, increased ROS generation and DNA fragmentation, disrupted mitochondrial membrane potential, and induced apoptotic cell death. However, TFCC pretreatment suppressed all of these adverse effects of doxorubicin. Signal transduction studies indicated that TFCC suppressed DOX-induced overexpression of p53 and phosphorylation of JNK, p38, and ERK. Studies with LY294002 (a PI3K/AKT inhibitor) demonstrated that the mechanism of TFCC-induced cardioprotection also involves activation of PI3K/AKT. These findings indicated the potential clinical application of TFCC in preventing DOX-induced cardiac oxidative stress.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Taurine suppresses doxorubicin-triggered oxidative stress and cardiac apoptosis in rat via up-regulation of PI3-K/Akt and inhibition of p53, p38-JNK.
- Record: found
- Abstract: found
- Article: not found
The protective role of arjunolic acid against doxorubicin induced intracellular ROS dependent JNK-p38 and p53-mediated cardiac apoptosis.
- Record: found
- Abstract: found
- Article: not found