- Record: found
- Abstract: found
- Article: found
Arabidopsis GAAPs interacting with MAPR3 modulate the IRE1-dependent pathway upon endoplasmic reticulum stress
Read this article at
Abstract
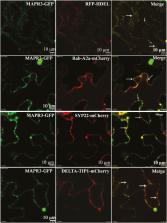
Arabidopsis GAAP1 and GAAP3 interacted with MAPR3. MAPR3 and the interaction between GAAPs and MAPR3 mitigated ER stress through modulation of RIDD and the autophagy pathway dependent on association with IRE1B.
Abstract
Cell viability requires the maintenance of intracellular homeostasis through the unfolded protein response mediated by receptors localized on the endoplasmic reticulum (ER) membrane. The receptor IRE1 mediates not only various adaptive outputs but also programmed cell death (PCD) under varying stress levels. However, little is known about the mechanism by which the same receptors trigger different responses in plants. Arabidopsis Golgi anti-apoptotic protein 1 (GAAP1) and GAAP3 resist PCD upon ER stress and negatively modulate the adaptive response of the IRE1–bZIP60 pathway through IRE1 association. To elucidate the mechanism underlying the anti-PCD activity of GAAPs, we attempted to isolate interactors of GAAPs by yeast two-hybrid screening. Membrane-associated progesterone receptor 3 (MAPR3) was isolated as one of the factors interacting with GAAP. Mutations in GAAP1/GAAP3 and/or MAPR3 enhanced the sensitivity of seedlings to ER stress. Whole-transcriptome analysis combined with quantitative reverse transcription–PCR and cellular analysis showed that regulated IRE1-dependent decay (RIDD) and autophagy were impaired in mutants mapr3, gaap1mapr3, and gaap3mapr3. MAPR3, GAAP1, and GAAP3 interacted with IRE1B as determined by protein interaction assays. These data suggest that the interaction of GAAP1/GAAP3 with MAPR3 mitigates ER stress to some extent through regulating IRE10-mediated RIDD and autophagy.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Autophagy: The Master of Bulk and Selective Recycling.
- Record: found
- Abstract: found
- Article: not found
Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha.
- Record: found
- Abstract: found
- Article: not found