- Record: found
- Abstract: found
- Article: found
Mucosal Immunity and Protective Efficacy of Intranasal Inactivated Influenza Vaccine Is Improved by Chitosan Nanoparticle Delivery in Pigs
Read this article at
Abstract
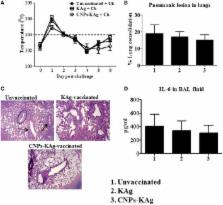
Annually, swine influenza A virus (SwIAV) causes severe economic loss to swine industry. Currently used inactivated SwIAV vaccines administered by intramuscular injection provide homologous protection, but limited heterologous protection against constantly evolving field viruses, attributable to the induction of inadequate levels of mucosal IgA and cellular immune responses in the respiratory tract. A novel vaccine delivery platform using mucoadhesive chitosan nanoparticles (CNPs) administered through intranasal (IN) route has the potential to elicit strong mucosal and systemic immune responses in pigs. In this study, we evaluated the immune responses and cross-protective efficacy of IN chitosan encapsulated inactivated SwIAV vaccine in pigs. Killed SwIAV H1N2 (δ-lineage) antigens (KAg) were encapsulated in chitosan polymer-based nanoparticles (CNPs-KAg). The candidate vaccine was administered twice IN as mist to nursery pigs. Vaccinates and controls were then challenged with a zoonotic and virulent heterologous SwIAV H1N1 (γ-lineage). Pigs vaccinated with CNPs-KAg exhibited an enhanced IgG serum antibody and mucosal secretory IgA antibody responses in nasal swabs, bronchoalveolar lavage (BAL) fluids, and lung lysates that were reactive against homologous (H1N2), heterologous (H1N1), and heterosubtypic (H3N2) influenza A virus strains. Prior to challenge, an increased frequency of cytotoxic T lymphocytes, antigen-specific lymphocyte proliferation, and recall IFN-γ secretion by restimulated peripheral blood mononuclear cells in CNPs-KAg compared to control KAg vaccinates were observed. In CNPs-KAg vaccinated pigs challenged with heterologous virus reduced severity of macroscopic and microscopic influenza-associated pulmonary lesions were observed. Importantly, the infectious SwIAV titers in nasal swabs [days post-challenge (DPC) 4] and BAL fluid (DPC 6) were significantly ( p < 0.05) reduced in CNPs-KAg vaccinates but not in KAg vaccinates when compared to the unvaccinated challenge controls. As well, an increased frequency of T helper memory cells and increased levels of recall IFNγ secretion by tracheobronchial lymph nodes cells were observed. In summary, chitosan SwIAV nanovaccine delivered by IN route elicited strong cross-reactive mucosal IgA and cellular immune responses in the respiratory tract that resulted in a reduced nasal viral shedding and lung virus titers in pigs. Thus, chitosan-based influenza nanovaccine may be an ideal candidate vaccine for use in pigs, and pig is a useful animal model for preclinical testing of particulate IN human influenza vaccines.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Molecular basis for the generation in pigs of influenza A viruses with pandemic potential.
- Record: found
- Abstract: found
- Article: found