- Record: found
- Abstract: found
- Article: found
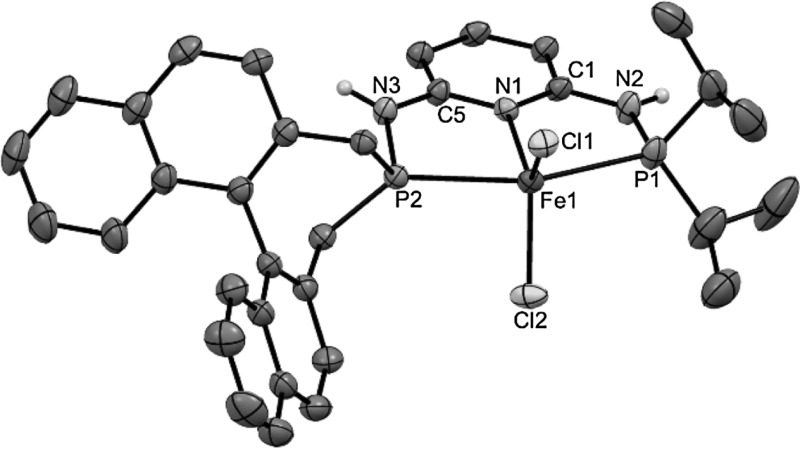
Synthesis and reactivity of BINEPINE-based chiral Fe(II) PNP pincer complexes

Read this article at
Abstract
Abstract
A new asymmetric chiral PNP ligand based on the 2,6-diaminopyridine scaffold featuring a R-BINEPINE moiety was prepared. Treatment of anhydrous FeX 2 (X = Cl, Br) with 1 equiv of PNP- iPr,BIN at room temperature afforded the coordinatively unsaturated paramagnetic complexes [Fe(PNP- iPr,BIN)X 2]. The structure of [Fe(PNP- iPr,BIN)Cl 2] is described. Both complexes react readily with the strong π-acceptor ligand CO in solution to afford selectively the diamagnetic complexes trans-[Fe(PNP- iPr,BIN)(CO)X 2] in quantitative yield. Due the lability of the CO ligand, these complexes are only stable under a CO atmosphere and isolation in pure form was not possible. The preparation of the carbonyl hydride complex [Fe(PNP- iPr,BIN)(H)(CO)Br] was achieved albeit in low yields via a one pot procedure by treatment of [Fe(PNP- iPr,BINEP)Br 2] with CO and subsequent reaction with Na[HBEt 3]. This complex was obtained as an inseparable mixture of two diastereomers in a ca. 1:1 ratio and was tested as catalyst for the hydrogenation of ketones. The catalyst showed acceptable activity under mild conditions (5 bar H 2, room temperature) with yields up to >99 % within 18 h.
Related collections
Most cited references49
- Record: found
- Abstract: not found
- Article: not found
Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density
- Record: found
- Abstract: not found
- Article: not found
Mercury: visualization and analysis of crystal structures
- Record: found
- Abstract: not found
- Article: not found
Iron catalysis in organic synthesis.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.