- Record: found
- Abstract: found
- Article: found
Mitochondrial Dysfunctions Contribute to Hypertrophic Cardiomyopathy in Patient iPSC-Derived Cardiomyocytes with MT-RNR2 Mutation
Read this article at
Summary
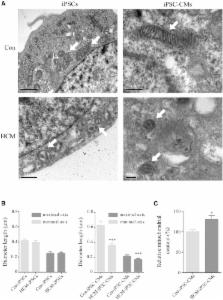
Hypertrophic cardiomyopathy (HCM) is the most common cause of sudden cardiac death in young individuals. A potential role of mtDNA mutations in HCM is known. However, the underlying molecular mechanisms linking mtDNA mutations to HCM remain poorly understood due to lack of cell and animal models. Here, we generated induced pluripotent stem cell-derived cardiomyocytes (HCM-iPSC-CMs) from human patients in a maternally inherited HCM family who carry the m.2336T>C mutation in the mitochondrial 16S rRNA gene ( MT-RNR2). The results showed that the m.2336T>C mutation resulted in mitochondrial dysfunctions and ultrastructure defects by decreasing the stability of 16S rRNA, which led to reduced levels of mitochondrial proteins. The ATP/ADP ratio and mitochondrial membrane potential were also reduced, thereby elevating the intracellular Ca 2+ concentration, which was associated with numerous HCM-specific electrophysiological abnormalities. Our findings therefore provide an innovative insight into the pathogenesis of maternally inherited HCM.
Highlights
Abstract
In this article, Yan Q, Liu Z, Huang W, and colleagues show that patient-specific iPSCs as well as their derived cardiomyocytes carrying the m.2336T>C mutation in MT-RNR2 were generated to understand the pathogenic mechanism of maternally inherited HCM. MT-RNR2 mutation resulted in mitochondrial dysfunctions and ultrastructure defects, which induced abnormal Ca 2+ homeostasis, then HCM-specific cellular and electrophysiological characteristics in iPSC-CMs.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches.
- Record: found
- Abstract: found
- Article: not found
Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy.
- Record: found
- Abstract: found
- Article: not found