- Record: found
- Abstract: found
- Article: found
LMP1-mediated glycolysis induces myeloid-derived suppressor cell expansion in nasopharyngeal carcinoma
Read this article at
Abstract
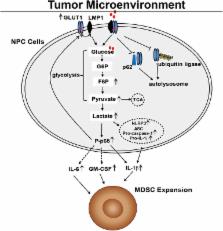
Myeloid-derived suppressor cells (MDSCs) are expanded in tumor microenvironments, including that of Epstein–Barr virus (EBV)-associated nasopharyngeal carcinoma (NPC). The link between MDSC expansion and EBV infection in NPC is unclear. Here, we show that EBV latent membrane protein 1 (LMP1) promotes MDSC expansion in the tumor microenvironment by promoting extra-mitochondrial glycolysis in malignant cells, which is a scenario for immune escape initially suggested by the frequent, concomitant detection of abundant LMP1, glucose transporter 1 (GLUT1) and CD33 + MDSCs in tumor sections. The full process has been reconstituted in vitro. LMP1 promotes the expression of multiple glycolytic genes, including GLUT1. This metabolic reprogramming results in increased expression of the Nod-like receptor family protein 3 (NLRP3) inflammasome, COX-2 and P-p65 and, consequently, increased production of IL-1β, IL-6 and GM-CSF. Finally, these changes in the environment of malignant cells result in enhanced NPC-derived MDSC induction. One key step is the physical interaction of LMP1 with GLUT1 to stabilize the GLUT1 protein by blocking its K48-ubiquitination and p62-dependent autolysosomal degradation. This work indicates that LMP1-mediated glycolysis regulates IL-1β, IL-6 and GM-CSF production through the NLRP3 inflammasome, COX-2 and P-p65 signaling pathways to enhance tumor-associated MDSC expansion, which leads to tumor immunosuppression in NPC.
Author summary
The expression of the Epstein-Barr virus (EBV) oncogenic protein denoted latent membrane protein 1 (LMP1) varies in patients with NPC and is linked to tumorigenesis and tumor immunosuppression, but the molecular mechanism through which LMP1 leads to tumor immune escape remains unknown. Work to date suggests that the expansion of tumor-associated myeloid-derived suppressor cells (MDSCs) is the main cause of tumor immunosuppression such as that found in NPC. Here, we found that tumor LMP1 expression is correlated with glucose transporter 1 (GLUT1) levels, CD33 + MDSC number and unfavorable survival in patients with NPC. Based on the results of our in vitro analysis, LMP1 promotes GLUT1-dependent glycolysis in NPC cells, resulting in activation of the Nod-like receptor family protein 3 (NLRP3) inflammasome, COX-2 and P-p65 signaling pathways and subsequently increased IL-1β, IL-6 and GM-CSF production. Importantly, LMP1 interacts with GLUT1 to stabilize the GLUT1 protein by disrupting its K48-linked ubiquitination and autolysosomal degradation in a p62-dependent manner and up-regulating the GLUT1 mRNA and protein levels by inducing p65 activation. Therefore, we determined that GLUT1-dependent glycolysis is required for tumor-induced MDSC differentiation and that this process is associated with LMP1 expression. Based on our findings, LMP1-mediated glycolysis is a key process involved in controlling tumor immunosuppression and directly contributes to oncogenesis.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes.
- Record: found
- Abstract: found
- Article: not found
Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells.
- Record: found
- Abstract: found
- Article: found