- Record: found
- Abstract: found
- Article: not found
Secretion of laccase and manganese peroxidase by Pleurotus strains cultivate in solid-state using Pinus spp. sawdust
Read this article at
Abstract
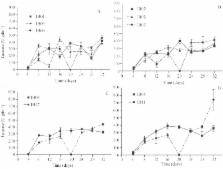
Pleurotus species secrete phenol oxidase enzymes: laccase (Lcc) and manganese peroxidase (MnP). New genotypes of these species show potential to be used in processes aiming at the degradation of phenolic compounds, polycyclic aromatic hydrocarbons and dyes. Hence, a screening of some strains of Pleurotus towards Lcc and MnP production was performed in this work. Ten strains were grown through solid-state fermentation on a medium based on Pinus spp. sawdust, wheat bran and calcium carbonate. High Lcc and MnP activities were found with these strains. Highest Lcc activity, 741 ± 245 U gdm −1 of solid state-cultivation medium, was detected on strain IB11 after 32 days, while the highest MnP activity occurred with strains IB05, IB09, and IB11 (5,333 ± 357; 4,701 ± 652; 5,999 ± 1,078 U gdm −1, respectively). The results obtained here highlight the importance of further experiments with lignocellulolytic enzymes present in different strains of Pleurotus species. Such results also indicate the possibility of selecting more valuable strains for future biotechnological applications, in soil bioremediation and biological biomass pre-treatment in biofuels production, for instance, as well as obtaining value-added products from mushrooms, like phenol oxidase enzymes.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Lentinus edodes and Pleurotus species lignocellulolytic enzymes activity in submerged and solid-state fermentation of lignocellulosic wastes of different composition.
- Record: found
- Abstract: found
- Article: not found
Influence of the carbohydrate moiety on the stability of glycoproteins.
- Record: found
- Abstract: not found
- Article: not found
