- Record: found
- Abstract: found
- Article: found
Epitope mapping of anti-drug antibodies to a clinical candidate bispecific antibody
Read this article at
ABSTRACT
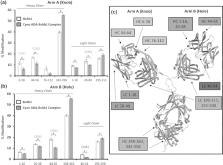
Anti-drug antibodies (ADA) can limit the efficacy and safety of therapeutic antibodies. However, determining the exact nature of ADA interactions with the target drug via epitope mapping is challenging due to the polyclonal nature of the IgG response. Here, we demonstrate successful proof-of-concept for the application of hydroxyl radical footprinting (HRF)-mass spectrometry for epitope mapping of ADAs obtained from goats that were administered a knob-into-hole bispecific antibody (BsAb1). Subsequently, we performed epitope mapping of ADAs obtained from cynomolgus (cyno) monkeys that were administered BsAb1 as we described in a recently published paper. Herein, we provide the first data to demonstrate the feasibility of using HRF for ADA epitope mapping, and show that both goat and cyno-derived ADAs specifically target the complementary-determining regions in both arms of BsAb1, suggesting that the ADA epitopes on BsAb1 may be species-independent.
Related collections
Most cited references25
- Record: found
- Abstract: not found
- Article: not found
‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization
- Record: found
- Abstract: found
- Article: not found
Immunogenicity of Biologics in Chronic Inflammatory Diseases: A Systematic Review
- Record: found
- Abstract: found
- Article: not found