- Record: found
- Abstract: found
- Article: found
Gephyrin, the enigmatic organizer at GABAergic synapses
Read this article at
Abstract
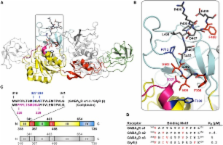
GABA A receptors are clustered at synaptic sites to achieve a high density of postsynaptic receptors opposite the input axonal terminals. This allows for an efficient propagation of GABA mediated signals, which mostly result in neuronal inhibition. A key organizer for inhibitory synaptic receptors is the 93 kDa protein gephyrin that forms oligomeric superstructures beneath the synaptic area. Gephyrin has long been known to be directly associated with glycine receptor β subunits that mediate synaptic inhibition in the spinal cord. Recently, synaptic GABA A receptors have also been shown to directly interact with gephyrin and interaction sites have been identified and mapped within the intracellular loops of the GABA A receptor α1, α2, and α3 subunits. Gephyrin-binding to GABA A receptors seems to be at least one order of magnitude weaker than to glycine receptors (GlyRs) and most probably is regulated by phosphorylation. Gephyrin not only has a structural function at synaptic sites, but also plays a crucial role in synaptic dynamics and is a platform for multiple protein-protein interactions, bringing receptors, cytoskeletal proteins and downstream signaling proteins into close spatial proximity.
Related collections
Most cited references151
- Record: found
- Abstract: found
- Article: not found
GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition.
- Record: found
- Abstract: found
- Article: not found
Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin.
- Record: found
- Abstract: found
- Article: not found