- Record: found
- Abstract: found
- Article: found
Cholinesterase and Prolyl Oligopeptidase Inhibitory Activities of Alkaloids from Argemone platyceras (Papaveraceae)
Read this article at
Abstract
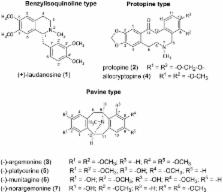
Alzheimer’s disease is an age-related, neurodegenerative disorder, characterized by cognitive impairment and restrictions in activities of daily living. This disease is the most common form of dementia with complex multifactorial pathological mechanisms. Many therapeutic approaches have been proposed. Among them, inhibition of acetylcholinesterase, butyrylcholinesterase, and prolyl oligopeptidase can be beneficial targets in the treatment of Alzheimer’s disease. Roots, along with aerial parts of Argemone platyceras, were extracted with ethanol and fractionated on an alumina column using light petrol, chloroform and ethanol. Subsequently, repeated preparative thin-layer chromatography led to the isolation of (+)-laudanosine, protopine, (–)-argemonine, allocryptopine, (–)-platycerine, (–)-munitagine, and (–)-norargemonine belonging to pavine, protopine and benzyltetrahydroisoquinoline structural types. Chemical structures of the isolated alkaloids were elucidated by optical rotation, spectroscopic and spectrometric analysis (NMR, MS), and comparison with literature data. (+)-Laudanosine was isolated from A. platyceras for the first time. Isolated compounds were tested for human blood acetylcholinesterase, human plasma butyrylcholinesterase and recombinant prolyl oligopeptidase inhibitory activity. The alkaloids inhibited the enzymes in a dose-dependent manner. The most active compound (–)-munitagine, a pavine alkaloid, inhibited both acetylcholinesterase and prolyl oligopeptidase with IC 50 values of 62.3 ± 5.8 µM and 277.0 ± 31.3 µM, respectively.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia.
- Record: found
- Abstract: found
- Article: not found
Cholinesterase inhibitors: new roles and therapeutic alternatives.
- Record: found
- Abstract: found
- Article: not found