- Record: found
- Abstract: found
- Article: found
Respiratory Tract Infections in Patients With Inflammatory Bowel Disease: Safety Analyses From Vedolizumab Clinical Trials
Read this article at
Abstract
Background and Aims
Vedolizumab, a humanised monoclonal antibody for the treatment of inflammatory bowel disease, selectively blocks gut lymphocyte trafficking. This may reduce the risk of respiratory tract infections [RTIs] compared with systemic immunosuppressive therapies. To assess this possibility, we evaluated the rates of RTIs in clinical trials of vedolizumab.
Methods
Patient-level data from Phase 3 randomised controlled trials [RCTs] of vedolizumab in ulcerative colitis [UC; GEMINI 1] and Crohn’s disease [CD; GEMINI 2], and a long-term safety study [UC and CD] were pooled. Cox proportional hazards models were used to estimate the incidence of upper RTIs [URTIs] and lower RTIs [LRTIs] with adjustment for significant covariates.
Results
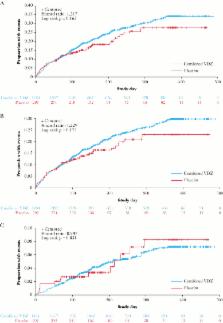
In the RCTs [ n = 1731 patients], the incidence of URTIs was numerically higher in patients receiving vedolizumab compared with those receiving placebo, although this difference was not statistically significant (38.7 vs 33.0 patients per 100 patient-years; hazard ratio [HR] 1.12; 95% confidence interval [CI]: 0.83–1.51; p = 0.463). The rate of LRTIs, including pneumonia, was numerically lower in the vedolizumab versus the placebo group: this difference was not statistically significant (7.7 vs 8.5 per 100 patient-years [HR 0.85; 95% CI: 0.48–1.52; p = 0.585]). Both URTIs and LRTIs were more frequent in patients with CD compared with UC. Most RTIs in patients receiving vedolizumab were not serious and did not require treatment discontinuation.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Infliximab for induction and maintenance therapy for ulcerative colitis.
- Record: found
- Abstract: found
- Article: not found
3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management.
- Record: found
- Abstract: found
- Article: not found