- Record: found
- Abstract: found
- Article: found
Activity-dependent somatodendritic dopamine release in the substantia nigra autoinhibits the releasing neuron

Read this article at
SUMMARY
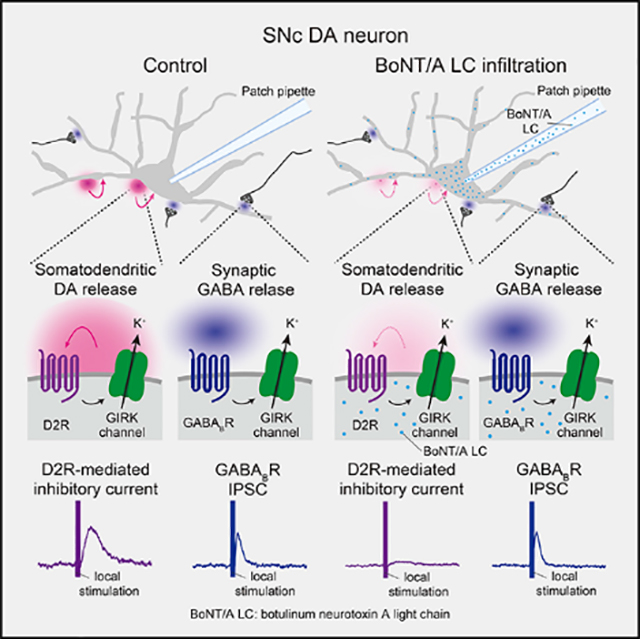
Somatodendritic dopamine (DA) release from midbrain DA neurons activates D2 autoreceptors on these cells to regulate their activity. However, the source of autoregulatory DA remains controversial. Here, we test the hypothesis that D2 autoreceptors on a given DA neuron in the substantia nigra pars compacta (SNc) are activated primarily by DA released from that same cell, rather than from its neighbors. Voltage-clamp recording allows monitoring of evoked D2-receptor-mediated inhibitory currents (D2ICs) in SNc DA neurons as an index of DA release. Single-cell application of antibodies to Na + channels via the recording pipette decreases spontaneous activity of recorded neurons and attenuates evoked D2ICs; antibodies to SNAP-25, a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein, also decrease D2IC amplitude. Evoked D2ICs are nearly abolished by the light chain of botulinum neurotoxin A, which cleaves SNAP-25, whereas synaptically activated GABA B-receptor-mediated currents are unaffected. Thus, somatodendritic DA release in the SNc autoinhibits the neuron that releases it.
In brief
Somatodendritic dopamine (DA) release inhibits DA neuron activity via D2 autoreceptors. A prevailing hypothesis is that this is mediated by synaptic release from neighboring DA neurons. Hikima et al. show instead that a given SNc DA neuron is regulated by its own released DA, thus exhibiting true autoinhibition.
Graphical Abstract
Related collections
Most cited references94
- Record: found
- Abstract: found
- Article: not found
Modulation of striatal projection systems by dopamine.
- Record: found
- Abstract: found
- Article: not found
The functional anatomy of basal ganglia disorders.
- Record: found
- Abstract: found
- Article: not found