- Record: found
- Abstract: found
- Article: found
Size-dependent gold nanoparticles induce macrophage M2 polarization and promote intracellular clearance of Staphylococcus aureus to alleviate tissue infection
Read this article at
Abstract
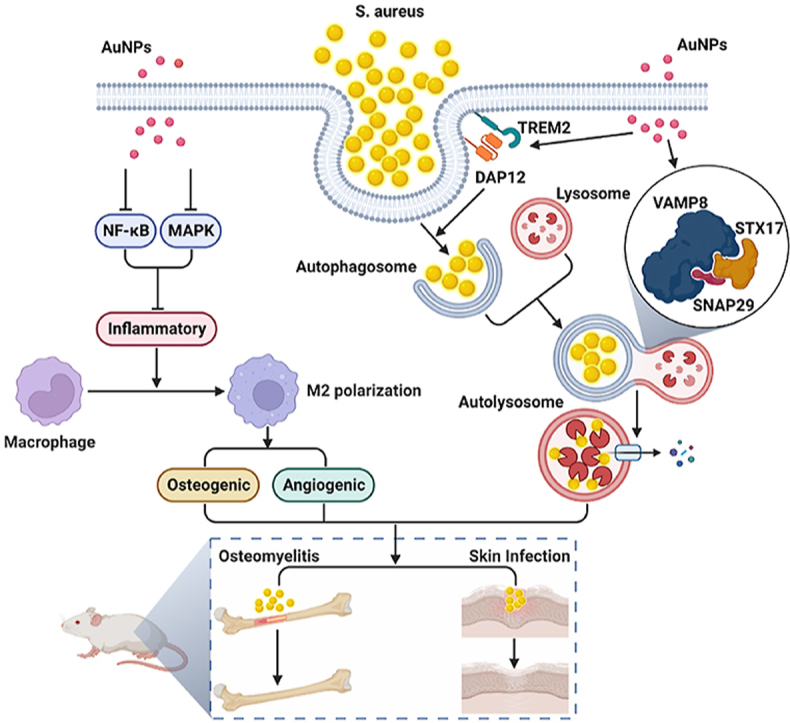
Tissue infection typically results from blood transmission or the direct inoculation of bacteria following trauma. The pathogen-induced destruction of tissue prevents antibiotics from penetrating the infected site, and severe inflammation further impairs the efficacy of conventional treatment. The current study describes the size-dependent induction of macrophage polarization using gold nanoparticles. Gold nanoparticles with a diameter of 50 nm (Au50) can induce M2 polarization in macrophages by inhibiting the NF-κB signaling pathway and stimulate an inflammatory response in the environment by inhibiting the MAPK signaling pathway LPS. Furthermore, the induced polarization and anti-inflammatory effects of the Au50 nanoparticles promoted the osteogenic differentiation of BMSCs in vitro. In addition, the overexpression of TREM2 in macrophage induced by Au50 nanoparticles was found to promote macrophage phagocytosis of Staphylococcus aureus, enhance the fusion of autophagosomes and lysosomes, accelerate the intracellular degradation of S. aureus, in addition to achieving an effective local treatment of osteomyelitis and infectious skin defects in conjunction with inflammatory regulation and accelerating bone regeneration. The findings, therefore, demonstrate that Au50 nanoparticles can be utilized as a promising nanomaterial for in vivo treatment of infections.
Graphical abstract
The graphical abstract was created with BioRender.com and the agreement number of confirmation of publication and licensing rights was EP25A4LE7Q.
Highlights
-
•
Au50 induces macrophage M2 polarization and attenuates LPS stimulated inflammatory response.
-
•
RNA-seq analysis of changes in whole genome expression of macrophages before and after the interaction of Au50 and LPS.
-
•
Au50 enhances bacterial phagocytosis by inducing overexpression of TREM2 in macrophages.
-
•
Au50 increases bacterial intracellular degradation in macrophages by promoting the fusion of autophagosomes and lysosomes.
-
•
Au50 accelerates the repair of infectious tissue defects in vivo.
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
Monocyte recruitment during infection and inflammation.
- Record: found
- Abstract: found
- Article: not found
The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes.

- Record: found
- Abstract: found
- Article: found