- Record: found
- Abstract: found
- Article: found
Current applications and future perspective of CRISPR/Cas9 gene editing in cancer
Read this article at
Abstract
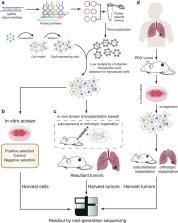
Clustered regularly interspaced short palindromic repeats (CRISPR) system provides adaptive immunity against plasmids and phages in prokaryotes. This system inspires the development of a powerful genome engineering tool, the CRISPR/CRISPR-associated nuclease 9 (CRISPR/Cas9) genome editing system. Due to its high efficiency and precision, the CRISPR/Cas9 technique has been employed to explore the functions of cancer-related genes, establish tumor-bearing animal models and probe drug targets, vastly increasing our understanding of cancer genomics. Here, we review current status of CRISPR/Cas9 gene editing technology in oncological research. We first explain the basic principles of CRISPR/Cas9 gene editing and introduce several new CRISPR-based gene editing modes. We next detail the rapid progress of CRISPR screening in revealing tumorigenesis, metastasis, and drug resistance mechanisms. In addition, we introduce CRISPR/Cas9 system delivery vectors and finally demonstrate the potential of CRISPR/Cas9 engineering to enhance the effect of adoptive T cell therapy (ACT) and reduce adverse reactions.
Related collections
Most cited references220
- Record: found
- Abstract: found
- Article: not found
A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity.
- Record: found
- Abstract: found
- Article: not found