- Record: found
- Abstract: found
- Article: not found
Cell size and invasion in TGF-β–induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway
Read this article at
Abstract
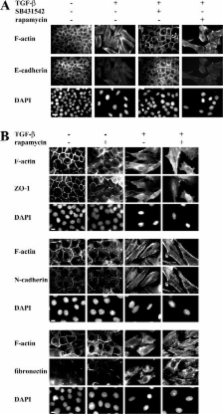
Epithelial to mesenchymal transition (EMT) occurs during development and cancer progression to metastasis and results in enhanced cell motility and invasion. Transforming growth factor-β (TGF-β) induces EMT through Smads, leading to transcriptional regulation, and through non-Smad pathways. We observe that TGF-β induces increased cell size and protein content during EMT. This translational regulation results from activation by TGF-β of mammalian target of rapamycin (mTOR) through phosphatidylinositol 3-kinase and Akt, leading to the phosphorylation of S6 kinase 1 and eukaryotic initiation factor 4E–binding protein 1, which are direct regulators of translation initiation. Rapamycin, a specific inhibitor of mTOR complex 1, inhibits the TGF-β–induced translation pathway and increase in cell size without affecting the EMT phenotype. Additionally, rapamycin decreases the migratory and invasive behavior of cells that accompany TGF-β–induced EMT. The TGF-β–induced translation pathway through mTOR complements the transcription pathway through Smads. Activation of mTOR by TGF-β, which leads to increased cell size and invasion, adds to the role of TGF-β–induced EMT in cancer progression and may represent a therapeutic opportunity for rapamycin analogues in cancer.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Neurotransmitter synthesis and uptake by isolated sympathetic neurones in microcultures.
- Record: found
- Abstract: found
- Article: not found
Bacterially speaking.
- Record: found
- Abstract: not found
- Article: not found
