- Record: found
- Abstract: found
- Article: found
Hantavirus in African Wood Mouse, Guinea
brief-report
Boris Klempa
*
,
† ,
Elisabeth Fichet-Calvet
‡ ,
Emilie Lecompte
§ ,
Brita Auste
* ,
Vladimir Aniskin
¶ ,
Helga Meisel
* ,
Christiane Denys
‡ ,
Lamine Koivogui
# ,
Jan ter Meulen
§
,
1 ,
Detlev H. Krüger
*
,
May 2006
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
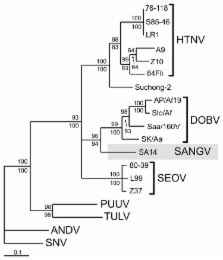
Hantaviruses are rodentborne, emerging viruses that cause life-threatening human diseases in Eurasia and the Americas. We detected hantavirus genome sequences in an African wood mouse ( Hylomyscus simus) captured in Sangassou, Guinea. Sequence and phylogenetic analyses of the genetic material demonstrate a novel hantavirus species, which we propose to name "Sangassou virus."
Related collections
Most cited references10
- Record: found
- Abstract: found
- Article: not found
Hantaviruses: a global disease problem.
C Schmaljohn, B Hjelle (1997)
- Record: found
- Abstract: found
- Article: not found
Persistent Sin Nombre virus infection in the deer mouse (Peromyscus maniculatus) model: sites of replication and strand-specific expression.
Chunyan Ye, Katy Mirowsky, Rebecca Gottlieb … (2002)