- Record: found
- Abstract: found
- Article: not found
Role of YidC in folding of polytopic membrane proteins
Read this article at
Abstract
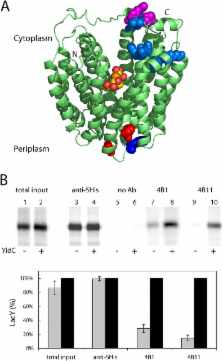
YidC of Echerichia coli, a member of the conserved Alb3/Oxa1/YidC family, is postulated to be important for biogenesis of membrane proteins. Here, we use as a model the lactose permease (LacY), a membrane transport protein with a known three-dimensional structure, to determine whether YidC plays a role in polytopic membrane protein insertion and/or folding. Experiments in vivo and with an in vitro transcription/translation/insertion system demonstrate that YidC is not necessary for insertion per se, but plays an important role in folding of LacY. By using the in vitro system and two monoclonal antibodies directed against conformational epitopes, LacY is shown to bind the antibodies poorly in YidC-depleted membranes. Moreover, LacY also folds improperly in proteoliposomes prepared without YidC. However, when the proteoliposomes are supplemented with purified YidC, LacY folds correctly. The results indicate that YidC plays a primary role in folding of LacY into its final tertiary conformation via an interaction that likely occurs transiently during insertion into the lipid phase of the membrane.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
X-ray structure of a protein-conducting channel.
- Record: found
- Abstract: found
- Article: not found
YidC mediates membrane protein insertion in bacteria.
- Record: found
- Abstract: found
- Article: not found
