- Record: found
- Abstract: found
- Article: found
Deficiency of Complement Component C1Q Prevents Cerebrovascular Damage and White Matter Loss in a Mouse Model of Chronic Obesity
Read this article at
Abstract
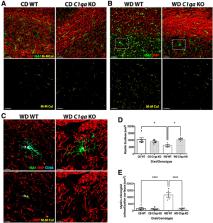
Age-related cognitive decline and many dementias involve complex interactions of both genetic and environmental risk factors. Recent evidence has demonstrated a strong association of obesity with the development of dementia. Furthermore, white matter damage is found in obese subjects and mouse models of obesity. Here, we found that components of the complement cascade, including complement component 1qa (C1QA) and C3 are increased in the brain of Western diet (WD)-fed obese mice, particularly in white matter regions. To functionally test the role of the complement cascade in obesity-induced brain pathology, female and male mice deficient in C1QA, an essential molecule in the activation of the classical pathway of the complement cascade, were fed a WD and compared with WD-fed wild type (WT) mice, and to C1qa knock-out (KO) and WT mice fed a control diet (CD). C1qa KO mice fed a WD became obese but did not show pericyte loss or a decrease in laminin density in the cortex and hippocampus that was observed in obese WT controls. Furthermore, obesity-induced microglia phagocytosis and breakdown of myelin in the corpus callosum were also prevented by deficiency of C1QA. Collectively, these data show that C1QA is necessary for damage to the cerebrovasculature and white matter damage in diet-induced obesity.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic.

- Record: found
- Abstract: found
- Article: found
White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities

- Record: found
- Abstract: found
- Article: found