- Record: found
- Abstract: found
- Article: found
Complement C1q-induced activation of β-catenin signalling causes hypertensive arterial remodelling
Read this article at
Abstract
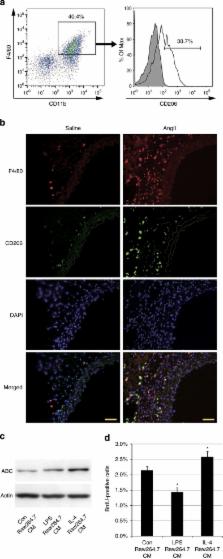
Hypertension induces structural remodelling of arteries, which leads to arteriosclerosis and end-organ damage. Hyperplasia of vascular smooth muscle cells (VSMCs) and infiltration of immune cells are the hallmark of hypertensive arterial remodelling. However, the precise molecular mechanisms of arterial remodelling remain elusive. We have recently reported that complement C1q activates β-catenin signalling independent of Wnts. Here, we show a critical role of complement C1-induced activation of β-catenin signalling in hypertensive arterial remodelling. Activation of β-catenin and proliferation of VSMCs were observed after blood-pressure elevation, which were prevented by genetic and chemical inhibition of β-catenin signalling. Macrophage depletion and C1qa gene deletion attenuated the hypertension-induced β-catenin signalling, proliferation of VSMCs and pathological arterial remodelling. Our findings unveil the link between complement C1 and arterial remodelling and suggest that C1-induced activation of β-catenin signalling becomes a novel therapeutic target to prevent arteriosclerosis in patients with hypertension.
Abstract
 The role of macrophages in hypertension-induced arterial remodeling is poorly understood.
Here, Sumida
et al. show that high blood pressure drives the alternatively activated macrophages to
secrete complement C1q protein, which in turn elicits proliferative β-catenin signalling
in the arterial smooth muscle cells.
The role of macrophages in hypertension-induced arterial remodeling is poorly understood.
Here, Sumida
et al. show that high blood pressure drives the alternatively activated macrophages to
secrete complement C1q protein, which in turn elicits proliferative β-catenin signalling
in the arterial smooth muscle cells.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Proximal events in Wnt signal transduction.
- Record: found
- Abstract: found
- Article: not found
Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies.
- Record: found
- Abstract: found
- Article: not found