- Record: found
- Abstract: found
- Article: found
Tendon stem cell-derived exosomes regulate inflammation and promote the high-quality healing of injured tendon
Read this article at
Abstract
Background
Tendon stem cells (TSCs) have been reported to hold promises for tendon repair and regeneration. However, less is known about the effects of exosomes derived from TSCs. Therefore, we aimed to clarify the healing effects of TSC-derived exosomes (TSC-Exos) on tendon injury.
Methods
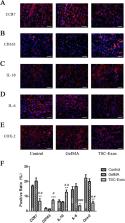
The Achilles tendons of Sprague-Dawley male rats were used for primary culture of TSCs and tenocytes, and exosomes were isolated from TSCs. The proliferation of tenocytes induced by TSC-Exos was analyzed using an EdU assay; cell migration was measured by cell scratch and transwell assays. We used western blot to analyze the role of the PI3K/AKT and MAPK/ERK1/2 signaling pathways. In vivo, Achilles tendon injury models were created in Sprague-Dawley rats. Rats ( n = 54) were then randomly assigned to three groups: the TSC-Exos group, the GelMA group, and the control group. We used immunofluorescence to detect changes in the expression of inflammatory and apoptotic markers at 1 week after surgery. Histology and changes in expression of extracellular matrix (ECM)-related indices were assessed by hematoxylin-eosin (H&E) staining and immunohistochemistry at 2 and 8 weeks. The collagen fiber diameter of the healing tendon was analyzed at 8 weeks by transmission electron microscopy (TEM).
Results
TSC-Exos were taken up by tenocytes, which promoted the proliferation and migration of cells in a dose-dependent manner; this process may depend on the activation of the PI3K/AKT and MAPK/ERK1/2 signaling pathways. At 1 week after surgery, we found that inflammation and apoptosis were significantly suppressed by TSC-Exos. At 2 and 8 weeks, tendons treated with TSC-Exos showed more continuous and regular arrangement in contrast to disorganized tendons in the GelMA and control groups, and TSC-Exos may help regulate ECM balance and inhibited scar formation. Further, at 8 weeks, the TSC-Exos group had a larger diameter of collagen compared to the control group.
Related collections
Most cited references42

- Record: found
- Abstract: found
- Article: found
Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis

- Record: found
- Abstract: found
- Article: found
Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts
- Record: found
- Abstract: found
- Article: not found