- Record: found
- Abstract: found
- Article: found
Tendon healing: a concise review on cellular and molecular mechanisms with a particular focus on the Achilles tendon
Read this article at
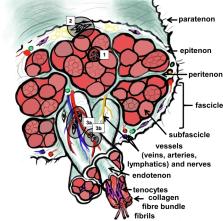
Abstract
Tendon is a bradytrophic and hypovascular tissue, hence, healing remains a major challenge. The molecular key events involved in successful repair have to be unravelled to develop novel strategies that reduce the risk of unfavourable outcomes such as non-healing, adhesion formation, and scarring. This review will consider the diverse pathophysiological features of tendon-derived cells that lead to failed healing, including misrouted differentiation (e.g. de- or transdifferentiation) and premature cell senescence, as well as the loss of functional progenitors. Many of these features can be attributed to disturbed cell-extracellular matrix (ECM) or unbalanced soluble mediators involving not only resident tendon cells, but also the cross-talk with immigrating immune cell populations. Unrestrained post-traumatic inflammation could hinder successful healing. Pro-angiogenic mediators trigger hypervascularization and lead to persistence of an immature repair tissue, which does not provide sufficient mechano-competence. Tendon repair tissue needs to achieve an ECM composition, structure, strength, and stiffness that resembles the undamaged highly hierarchically ordered tendon ECM. Adequate mechano-sensation and -transduction by tendon cells orchestrate ECM synthesis, stabilization by cross-linking, and remodelling as a prerequisite for the adaptation to the increased mechanical challenges during healing. Lastly, this review will discuss, from the cell biological point of view, possible optimization strategies for augmenting Achilles tendon (AT) healing outcomes, including adapted mechanostimulation and novel approaches by restraining neoangiogenesis, modifying stem cell niche parameters, tissue engineering, the modulation of the inflammatory cells, and the application of stimulatory factors.
Cite this article: Bone Joint Res 2022;11(8):561–574.
Related collections
Most cited references143
- Record: found
- Abstract: found
- Article: not found
Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche.

- Record: found
- Abstract: found
- Article: found
Senescence and the SASP: many therapeutic avenues
- Record: found
- Abstract: found
- Article: not found