- Record: found
- Abstract: found
- Article: found
Halide Superionic Conductors for All-Solid-State Batteries: Effects of Synthesis and Composition on Lithium-Ion Conductivity

Read this article at
Abstract
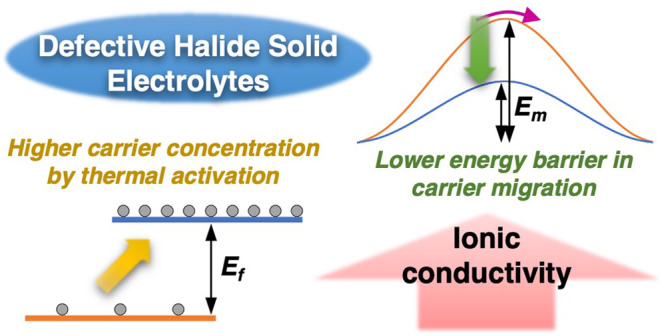
Owing to their high-voltage stabilities, halide superionic conductors such as Li 3YCl 6 recently emerged as promising solid electrolyte (SE) materials for all-solid-state batteries (ASSBs). It has been shown that by either introducing off-stoichiometry in solid-state (SS) synthesis or using a mechanochemical (MC) synthesis method the ionic conductivities of Li 3–3 x Y 1+ x Cl 6 can increase up to an order of magnitude. The underlying mechanism, however, is unclear. In the present study, we adopt a hopping frequency analysis method of impedance spectra to reveal the correlations in stoichiometry, crystal structure, synthesis conditions, Li + carrier concentrations, hopping migration barriers, and ionic conductivity. We show that unlike the conventional Li 3YCl 6 made by SS synthesis, mobile Li + carriers in the defect-containing SS-Li 3–3 x Y 1+ x Cl 6 (0 < x < 0.17) and MC-Li 3–3 x Y 1+ x Cl 6 are generated with an activation energy and their concentration is dependent on temperature. Higher ionic conductivities in these samples arise from a combination of a higher Li + carrier concentration and lower migration energy barriers. A new off-stoichiometric halide (Li 2.61Y 1.13Cl 6) with the highest ionic conductivity (0.47 mS cm –1) in the series is discovered, which delivers exceptional cycling performance (∼90% capacity retention after 1000 cycles) in ASSB cells equipped with an uncoated high-energy LiNi 0.8Mn 0.1Co 0.1O 2 (NMC811) cathode. This work sheds light on the thermal activation process that releases trapped Li + ions in defect-containing halides and provides guidance for the future development of superionic conductors for all-solid-state batteries.
Related collections
Most cited references55
- Record: found
- Abstract: not found
- Article: not found
Lithium battery chemistries enabled by solid-state electrolytes
- Record: found
- Abstract: found
- Article: not found
Inorganic Solid-State Electrolytes for Lithium Batteries: Mechanisms and Properties Governing Ion Conduction.
- Record: found
- Abstract: not found
- Article: not found