- Record: found
- Abstract: found
- Article: not found
NSAIDs Modulate CDKN2A, TP53, and DNA Content Risk for Progression to Esophageal Adenocarcinoma
Read this article at
Abstract
Background
Somatic genetic CDKN2A, TP53, and DNA content abnormalities are common in many human cancers and their precursors, including esophageal adenocarcinoma (EA) and Barrett's esophagus (BE), conditions for which aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) have been proposed as possible chemopreventive agents; however, little is known about the ability of a biomarker panel to predict progression to cancer nor how NSAID use may modulate progression. We aimed to evaluate somatic genetic abnormalities with NSAIDs as predictors of EA in a prospective cohort study of patients with BE.
Methods and Findings
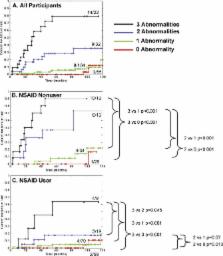
Esophageal biopsies from 243 patients with BE were evaluated at baseline for TP53 and CDKN2A (p16) alterations, tetraploidy, and aneuploidy using sequencing; loss of heterozygosity (LOH); methylation-specific PCR; and flow cytometry. At 10 y, all abnormalities, except CDKN2A mutation and methylation, contributed to EA risk significantly by univariate analysis, ranging from 17p LOH (relative risk [RR] = 10.6; 95% confidence interval [CI] 5.2–21.3, p < 0.001) to 9p LOH (RR = 2.6; 95% CI 1.1–6.0, p = 0.03). A panel of abnormalities including 17p LOH, DNA content tetraploidy and aneuploidy, and 9p LOH was the best predictor of EA (RR = 38.7; 95% CI 10.8–138.5, p < 0.001). Patients with no baseline abnormality had a 12% 10-y cumulative EA incidence, whereas patients with 17p LOH, DNA content abnormalities, and 9p LOH had at least a 79.1% 10-y EA incidence. In patients with zero, one, two, or three baseline panel abnormalities, there was a significant trend toward EA risk reduction among NSAID users compared to nonusers ( p = 0.01). The strongest protective effect was seen in participants with multiple genetic abnormalities, with NSAID nonusers having an observed 10-y EA risk of 79%, compared to 30% for NSAID users ( p < 0.001).
Abstract
In a ten-year study of people with Barrett's esophagus, nonsteroidal anti-inflamatory drugs were associated with reduced risk of esophageal adenocarcinoma, especially in patients with multiple high-risk molecular abnormalities.
Editors' Summary
Background.
Normally, the cells in the human body divide only when extra cells are needed, after an injury, for example. Sometimes, however, cells accumulate genetic changes (mutations) that allow them to divide uncontrollably to form a disorganized mass or tumor. If these altered cells also acquire mutations that allow them to spread around the body, a malignant tumor or cancer results. Scientists have identified numerous genetic changes that occur in tumors and are now investigating whether these molecular abnormalities can be used as “biomarkers” to choose the best treatments for patients, to identify who will benefit from cancer-prevention strategies, to detect cancer early, and to predict which cancers are most likely to become life-threatening. This last application is particularly important for cancers with a well-defined premalignant stage. Because the cells in premalignant tissues have acquired some of the genetic changes required for cancer development, they are more likely to become malignant than normal cells. Barrett's esophagus, for example, is a premalignant disorder of the muscular tube that takes food from the mouth to the stomach. People with Barrett's esophagus are much more likely to develop esophageal cancer than the general population.
Why Was This Study Done?
Esophageal cancer is often incurable by the time it is detected, so it would be helpful to know which people with Barrett's esophagus are most likely to develop esophageal cancer—only 1 in 200 of them develop cancer each year. In this study, the researchers evaluated whether a panel of genetic alterations could identify this subset of patients. They also investigated whether the regular use of aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) affects the risk of developing esophageal cancer in people with Barrett's esophagus—other evidence suggests that NSAIDs may help to prevent several types of cancer, including esophageal cancer.
What Did the Researchers Do and Find?
The researchers took esophageal tissue samples from patients with Barrett's esophagus and looked for alterations in the genes encoding the tumor-suppressor proteins TP53 and CDKN2A. These proteins normally stop cells dividing but are often inactivated in cancer cells by mutation of one of the two gene copies that encode each of them and also loss of the other copy (so-called “loss of heterozygosity” or LOH). The researchers also looked for changes in the cellular DNA content of the samples (tumor cells often contain unusual amounts of DNA) and asked the study participants about their NSAID use before waiting to see which participants developed esophageal cancer. After 10 y, the participants whose tissue samples had LOH of the short arms (p) of Chromosome 17 or 9 (the sites of the genes encoding TP53 and CDKN2A, respectively), or an altered DNA content, were more likely to have developed esophageal cancer than those without these abnormalities; those whose samples contained all three abnormalities had the highest risk of developing esophageal cancer. Overall, just 12% of patients with no abnormalities but nearly 80% of patients with three abnormalities developed esophageal cancer. NSAID use reduced the risk of cancer development in all the participants, but its effect was greatest in those with three genetic abnormalities.
What Do These Findings Mean?
These findings suggest that the combined measurement of 17pLOH, 9pLOH, and cellular DNA content might be a powerful way to identify those patients with Barrett's esophagus who are most likely to develop esophageal cancer. They also suggest that NSAID use is associated with a reduced risk of esophageal cancer, particularly in patients with multiple genetic abnormalities. Because very few participants developed cancer during the study, these results need confirming in more patients. Also, the ability of NSAIDs to prevent the progression of Barrett's esophagus to esophageal cancer needs testing in multicenter randomized trials; the use of the panel of abnormalities described here to identify the people with Barrett's esophagus most at risk of developing esophageal cancer should facilitate such studies.
Additional Information.
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.0040067.
-
CancerQuest information from Emory University (Atlanta, Georgia, United States) on cancer biology, including the role of tumor suppressor proteins
-
US National Cancer Institute patient and physician information on esophageal cancer and its prevention
-
MedlinePlus encyclopedia pages on Barrett's esophagus and esophageal cancer
-
Cancerbackup (UK charity) patient information on esophageal cancer and Barrett's esophagus
Related collections
Most cited references111
- Record: found
- Abstract: not found
- Article: not found
Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing
- Record: found
- Abstract: not found
- Article: not found
A genetic model for colorectal tumorigenesis.
- Record: found
- Abstract: found
- Article: not found
