- Record: found
- Abstract: found
- Article: found
Simple high-cell density fed-batch technique for high-level recombinant protein production with Pichia pastoris: Application to intracellular production of Hepatitis B surface antigen
Read this article at
Abstract
Background
Hepatitis B is a serious global public health concern. Though a safe and efficacious recombinant vaccine is available, its use in several resource-poor countries is limited by cost. We have investigated the production of Hepatitis B virus surface antigen (HBsAg) using the yeast Pichia pastoris GS115 by inserting the HBsAg gene into the alcohol oxidase 1 locus.
Results
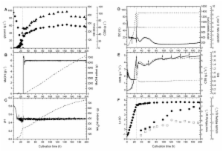
Large-scale production was optimized by developing a simple fed-batch process leading to enhanced product titers. Cells were first grown rapidly to high-cell density in a batch process using a simple defined medium with low salt and high glycerol concentrations. Induction of recombinant product synthesis was carried out using rather drastic conditions, namely through the addition of methanol to a final concentration of 6 g L -1. This methanol concentration was kept constant for the remainder of the cultivation through continuous methanol feeding based on the on-line signal of a flame ionization detector employed as methanol analyzer in the off-gas stream. Using this robust feeding protocol, maximum concentrations of ~7 grams HBsAg per liter culture broth were obtained. The amount of soluble HBsAg, competent for assembly into characteristic virus-like particles (VLPs), an attribute critical to its immunogenicity and efficacy as a hepatitis B vaccine, reached 2.3 grams per liter of culture broth.
Conclusion
In comparison to the highest yields reported so far, our simple cultivation process resulted in an ~7 fold enhancement in total HBsAg production with more than 30% of soluble protein competent for assembly into VLPs. This work opens up the possibility of significantly reducing the cost of vaccine production with implications for expanding hepatitis B vaccination in resource-poor countries.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Virus-like particles: Passport to immune recognition
- Record: found
- Abstract: found
- Article: not found
Synthesis and assembly of hepatitis B virus surface antigen particles in yeast.
- Record: found
- Abstract: found
- Article: not found