- Record: found
- Abstract: found
- Article: found
Hypoxia reduces testosterone synthesis in mouse Leydig cells by inhibiting NRF1-activated StAR expression
Read this article at
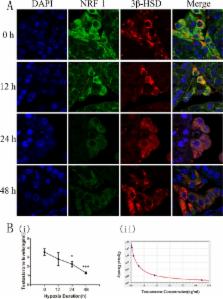
Abstract
Male fertility disorders play a key role in half of all infertility cases. Reduction in testosterone induced by hypoxia might cause diseases in reproductive system and other organs. Hypoxic exposure caused a significant decrease of NRF1. Software analysis reported that the promoter region of steroidogenic acute regulatory protein (StAR) contained NRF1 binding sites, indicating NRF1 promoted testicular steroidogenesis. The purpose of this study is to determine NRF1 is involved in testosterone synthesis; and under hypoxia, the decrease of testosterone synthesis is caused by lower expression of NRF1. We designed both in vivo and in vitro experiments. Under hypoxia, the expressions of NRF1 in Leydig cells and testosterone level were significantly decreased both in vivo and in vitro. Overexpression and interference NRF1 could induced StAR and testosterone increased and decreased respectively. ChIP results confirmed the binding of NRF1 to StAR promoter region. In conclusion, decline of NRF1 expression downregulated the level of StAR, which ultimately resulted in a reduction in testosterone synthesis.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
The life, death, and replacement of oligodendrocytes in the adult CNS.
- Record: found
- Abstract: found
- Article: not found