- Record: found
- Abstract: found
- Article: found
Sclareol and linalyl acetate are produced by glandular trichomes through the MEP pathway
Read this article at
Abstract
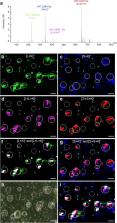
Sclareol, an antifungal specialized metabolite produced by clary sage, Salvia sclarea, is the starting plant natural molecule used for the hemisynthesis of the perfume ingredient ambroxide. Sclareol is mainly produced in clary sage flower calyces; however, the cellular localization of the sclareol biosynthesis remains unknown. To elucidate the site of sclareol biosynthesis, we analyzed its spatial distribution in the clary sage calyx epidermis using laser desorption/ionization mass spectrometry imaging (LDI–FTICR-MSI) and investigated the expression profile of sclareol biosynthesis genes in isolated glandular trichomes (GTs). We showed that sclareol specifically accumulates in GTs’ gland cells in which sclareol biosynthesis genes are strongly expressed. We next isolated a glabrous beardless mutant and demonstrate that more than 90% of the sclareol is produced by the large capitate GTs. Feeding experiments, using 1- 13C-glucose, and specific enzyme inhibitors further revealed that the methylerythritol-phosphate (MEP) biosynthetic pathway is the main source of isopentenyl diphosphate (IPP) precursor used for the biosynthesis of sclareol. Our findings demonstrate that sclareol is an MEP-derived diterpene produced by large capitate GTs in clary sage emphasing the role of GTs as biofactories dedicated to the production of specialized metabolites.
Related collections
Most cited references63
- Record: found
- Abstract: not found
- Article: not found
Factors affecting secondary metabolite production in plants: volatile components and essential oils
- Record: found
- Abstract: found
- Article: not found
Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent.
- Record: found
- Abstract: not found
- Article: not found