- Record: found
- Abstract: found
- Article: found
COX5B Regulates MAVS-mediated Antiviral Signaling through Interaction with ATG5 and Repressing ROS Production
Read this article at
Abstract
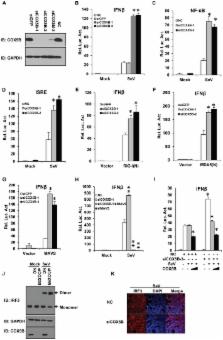
Innate antiviral immunity is the first line of the host defense system that rapidly detects invading viruses. Mitochondria function as platforms for innate antiviral signal transduction in mammals through the adaptor protein, MAVS. Excessive activation of MAVS-mediated antiviral signaling leads to dysfunction of mitochondria and cell apoptosis that likely causes the pathogenesis of autoimmunity. However, the mechanism of how MAVS is regulated at mitochondria remains unknown. Here we show that the Cytochrome c Oxidase (CcO) complex subunit COX5B physically interacts with MAVS and negatively regulates the MAVS-mediated antiviral pathway. Mechanistically, we find that while activation of MAVS leads to increased ROS production and COX5B expression, COX5B down-regulated MAVS signaling by repressing ROS production. Importantly, our study reveals that COX5B coordinates with the autophagy pathway to control MAVS aggregation, thereby balancing the antiviral signaling activity. Thus, our study provides novel insights into the link between mitochondrial electron transport system and the autophagy pathway in regulating innate antiviral immunity.
Author Summary
Pattern recognition receptors are vital to innate immunity. In the antiviral innate immunity, retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), such as RIG-I and MDA5, sense viral RNAs through their C-terminal helicase domains, then initiate the antiviral response through interaction with the essential adaptor protein MAVS, which is located in mitochondrial outer membrane. Although cumulative studies have showed that mitochondria-associated MAVS plays an important role in antiviral signaling, much remains unknown about the mechanism of MAVS activity related to mitochondrial membrane localization. In this article we demonstrate that the CcO complex subunit COX5B negatively regulates the MAVS-mediated antiviral pathway through interaction with MAVS. At the mechanistic level, we show that COX5B inhibits MAVS-mediated antiviral pathway by suppressing ROS production, and coordinating with the autophagy pathway to control MAVS aggregation. Our data support a notion that mitochondrial electron transport system coordinates with the autophagy pathway to regulate MAVS-mediated signaling for a tight control of innate antiviral immunity.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Type I interferons (alpha/beta) in immunity and autoimmunity.
- Record: found
- Abstract: found
- Article: not found
Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response.
- Record: found
- Abstract: found
- Article: not found