- Record: found
- Abstract: found
- Article: found
Targeted and redox-responsive drug delivery systems based on carbonic anhydrase IX-decorated mesoporous silica nanoparticles for cancer therapy
Read this article at
Abstract
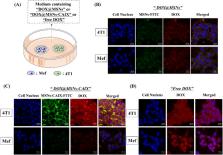
In this work, we developed a new antibody-targeted and redox-responsive drug delivery system “MSNs-CAIX” by binding the anti-carbonic anhydrase IX antibody (A-CAIX Ab) on the surface of mesoporous silica nanoparticles (MSNs) via disulfide linkages. The design of the composite particles “MSNs-CAIX” involved the synthesis and surface functionalization with thiol groups, 2,2′-dipyridyl disulfide and CAIX antibody. In vitro, CAIX capping the doxorubicin hydrochloric (DOX)-loaded nanoparticles (DOX@MSNs-CAIX) exhibited effectively redox-responsive release in the presence of glutathione (GSH) owing to the cleavage of the disulfide bond. Compared with CAIX negative Mef cells (mouse embryo fibroblast), remarkably more DOX@MSNs-CAIX was internalized into CAIX positive 4T1 cells (mouse breast cancer cells) by receptor-mediation. Tumor targeting in vivo studies clearly demonstrated DOX@MSNs-CAIX accumulated in tumors and induced more tumor cells apoptosis in 4T1 tumor-bearing mice. With great potential, this drug delivery system is a promising candidate for targeted and redox-responsive cancer therapy.
Related collections
Most cited references41

- Record: found
- Abstract: found
- Article: found
Progress and challenges towards targeted delivery of cancer therapeutics
- Record: found
- Abstract: found
- Article: not found
Functionalized mesoporous silica materials for controlled drug delivery.
- Record: found
- Abstract: found
- Article: found