- Record: found
- Abstract: found
- Article: found
Establishment of a Human Blood-Brain Barrier Co-culture Model Mimicking the Neurovascular Unit Using Induced Pluri- and Multipotent Stem Cells

Read this article at
Summary
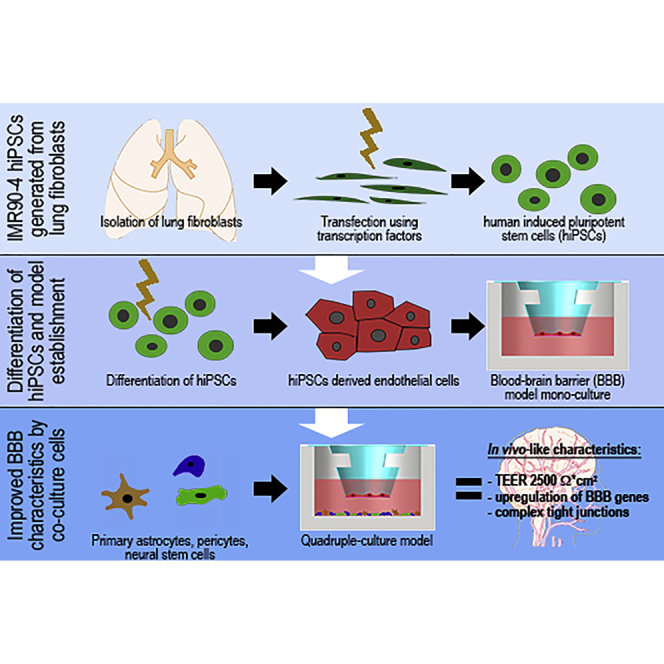
In vitro models of the human blood-brain barrier (BBB) are highly desirable for drug development. This study aims to analyze a set of ten different BBB culture models based on primary cells, human induced pluripotent stem cells (hiPSCs), and multipotent fetal neural stem cells (fNSCs). We systematically investigated the impact of astrocytes, pericytes, and NSCs on hiPSC-derived BBB endothelial cell function and gene expression. The quadruple culture models, based on these four cell types, achieved BBB characteristics including transendothelial electrical resistance (TEER) up to 2,500 Ω cm 2 and distinct upregulation of typical BBB genes. A complex in vivo-like tight junction (TJ) network was detected by freeze-fracture and transmission electron microscopy. Treatment with claudin-specific TJ modulators caused TEER decrease, confirming the relevant role of claudin subtypes for paracellular tightness. Drug permeability tests with reference substances were performed and confirmed the suitability of the models for drug transport studies.
Graphical Abstract
Highlights
-
•
Establishment of a standardized human BBB co-culture model based on hiPSCs and fNSCs
-
•
Reflection of physiological BBB integrity and expression of relevant transporters/TJs
-
•
Confirmation of TJ network functionality by claudin-specific TJ modulators
-
•
Validation of physiological transcellular model tightness by permeability studies
Abstract
In this article, Metzger and colleagues present the establishment of physiologically relevant human blood-brain barrier quadruple culture models based on induced pluripotent and multipotent stem cells. The novel model can be used as a powerful tool in pharmaceutical drug research and ADMET studies, which may allow a more reliable translation of promising drug candidates into clinical application.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study.
- Record: found
- Abstract: found
- Article: not found
Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology.

- Record: found
- Abstract: found
- Article: found