- Record: found
- Abstract: found
- Article: found
Analysis of Nurse and Patient Preferences for Pre-Filled Pen Devices for Self-Injection of Highly Purified Human Menopausal Gonadotropin (HP-hMG, MENOPUR ®)

Abstract
Purpose
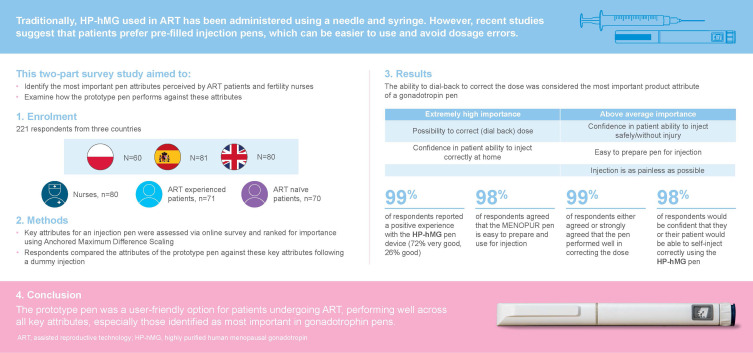
This study aimed to identify the most important attributes for a gonadotropin pen as perceived by assisted reproductive technology (ART) patients and fertility nurses, and to examine how well a prototype HP-hMG (MENOPUR ®) pen reflects these preferences.
Patients and Methods
This market research study incorporated a two-part survey with respondents (N=221) from Poland, Spain and the UK. Respondents included patients (n=141) who consulted a fertility specialist in the previous 2 years, and fertility nurses (n=80) who assisted in at least 75 ART cycles/year. Patients were divided into two subgroups depending on their experience with ART (experienced and naïve). Key attributes for an injection pen, as perceived by patients and nurses, were assessed via an online survey and ranked by their relative importance using Anchored Maximum Difference Scaling. After performing a dummy injection, respondents compared the attributes of an unbranded prototype pen against the key attributes identified.
Results
Across all survey respondents, the ability to correct the dialed dose was considered to be the most important product attribute of a gonadotropin pen. Confidence in the patient’s ability to inject correctly at home was also identified as a key attribute, considered by both nurses and naïve patients as extremely high. When considering the prototype pen device, almost all study respondents reported a positive experience (99%) with 72% rating it as “very good”. The prototype pen was perceived to possess the key attributes considered important for a gonadotropin pen by patients and nurses, including correcting the dose, the ability to self-inject safely and correctly, ease of preparation and use, and an injection which appeared to be as painless as possible.
Graphical Abstract
Video Abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Most cited references26
- Record: found
- Abstract: found
- Article: not found
A randomized assessor-blind trial comparing highly purified hMG and recombinant FSH in a GnRH antagonist cycle with compulsory single-blastocyst transfer.

- Record: found
- Abstract: found
- Article: found
The Development of Gonadotropins for Clinical Use in the Treatment of Infertility
- Record: found
- Abstract: found
- Article: not found