- Record: found
- Abstract: found
- Article: found
Immune checkpoint inhibitors in lymphoma: challenges and opportunities
Read this article at
Abstract
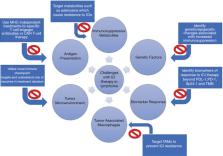
Immune checkpoint inhibitors (ICIs) are immunomodulatory antibodies that intensify the host immune response, thereby leading to cytotoxicity. The primary targets for checkpoint inhibition have included cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death receptor-1 (PD-1) or programmed cell death ligand-1 (PD-L1). ICIs have resulted in a change in treatment landscape of various neoplasms. Among hematologic malignancies, ICIs have been most successful in certain subtypes of lymphomas such as classic Hodgkin lymphoma (cHL) and primary mediastinal B-cell lymphoma (PMBCL). However, there have been several challenges in harnessing the host immune system through ICI use in other lymphomas. The underlying reasons for the low efficacy of ICI monotherapy in most lymphomas may include defects in antigen presentation, non-inflamed tumor microenvironment (TME), immunosuppressive metabolites, genetic factors, and an overall lack of predictive biomarkers of response. In this review, we outline the existing and ongoing studies utilizing ICI therapy in various lymphomas. We also describe the challenges leading to the lack of efficacy with ICI use and discuss potential strategies to overcome those challenges including: chimeric antigen receptor T-cell therapy (CAR-T therapy), bispecific T-cell therapy (BiTE), lymphocyte activation gene-3 (LAG-3) inhibitors, T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) inhibitors, vaccines, promotion of inflammatory macrophages, indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors, DNA methyltransferase inhibitors (DNMTi) and histone deacetylase inhibitors (HDACi). Tumor mutational burden and interferon-gamma release assays are potential biomarkers of ICI treatment response beyond PD-L1 expression. Further collaborations between clinicians and scientists are vital to understand the immunopathology in ICI therapy in order to improve clinical outcomes.
Related collections
Most cited references119
- Record: found
- Abstract: found
- Article: not found
Signatures of mutational processes in human cancer
- Record: found
- Abstract: found
- Article: not found
Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors
- Record: found
- Abstract: found
- Article: not found