- Record: found
- Abstract: found
- Article: found
The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation
Read this article at
Abstract
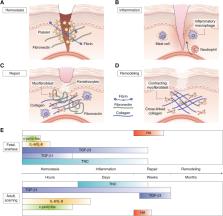
Various forms of fibrosis, comprising tissue thickening and scarring, are involved in 40% of deaths across the world. Since the discovery of scarless functional healing in fetuses prior to a certain stage of development, scientists have attempted to replicate scarless wound healing in adults with little success. While the extracellular matrix (ECM), fibroblasts, and inflammatory mediators have been historically investigated as separate branches of biology, it has become increasingly necessary to consider them as parts of a complex and tightly regulated system that becomes dysregulated in fibrosis. With this new paradigm, revisiting fetal scarless wound healing provides a unique opportunity to better understand how this highly regulated system operates mechanistically. In the following review, we navigate the four stages of wound healing (hemostasis, inflammation, repair, and remodeling) against the backdrop of adult versus fetal wound healing, while also exploring the relationships between the ECM, effector cells, and signaling molecules. We conclude by singling out recent findings that offer promising leads to alter the dynamics between the ECM, fibroblasts, and inflammation to promote scarless healing. One factor that promises to be significant is fibroblast heterogeneity and how certain fibroblast subpopulations might be predisposed to scarless healing. Altogether, reconsidering fetal wound healing by examining the interplay of the various factors contributing to fibrosis provides new research directions that will hopefully help us better understand and address fibroproliferative diseases, such as idiopathic pulmonary fibrosis, liver cirrhosis, systemic sclerosis, progressive kidney disease, and cardiovascular fibrosis.
Related collections
Most cited references313
- Record: found
- Abstract: found
- Article: not found
Neutrophil extracellular traps kill bacteria.
- Record: found
- Abstract: found
- Article: not found
Macrophages in Tissue Repair, Regeneration, and Fibrosis.
- Record: found
- Abstract: found
- Article: not found