- Record: found
- Abstract: found
- Article: found
Monitoring the efficacy of dendritic cell vaccination by early detection of 99mTc-HMPAO-labelled CD4 + T cells
research-article
Ehsan Sharif-Paghaleh
1
,
2 ,
John Leech
1 ,
Kavitha Sunassee
2 ,
Niwa Ali
1 ,
Pervinder Sagoo
1 ,
Robert I Lechler
1 ,
Lesley A Smyth
1 ,
Giovanna Lombardi
1 ,
Gregory E Mullen
1
,
2
19 March 2014
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
DC vaccines have been used to induce tumour-specific cytotoxic T cells 1. However,
this approach to cancer immunotherapy has had limited success. To be
successful, injected DCs need to migrate to the LNs where they can stimulate effector
T cells 1. We and others have previously demonstrated by MRI that tumour
antigen-pulsed-DCs labelled ex vivo with superparamagnetic iron oxide nanoparticles
migrated to the
draining LNs and are capable of activating antigen-specific T cells 2, 3. The results
from our study demonstrated that ex
vivo superparamagnetic iron oxide nanoparticles-labelled and OVA-pulsed DCs prime
cytotoxic
CD8+ T-cell responses to protect against a B16-OVA tumour challenge. In the
clinic, a possible noninvasive surrogate marker for efficacy of DC vaccination is
to image the
specific migration and accumulation of T cells following DC vaccination.
Mononuclear cells can be directly ex vivo radiolabelled with
99mTc-Hexamethylpropyleneamine oxime (99mTc-HMPAO) allowing the migratory
pathway of adoptively transferred cells to be tracked by single photon emission computed
tomography
CT (SPECT/CT) 4. Here, we combined our previous experience
with DC vaccination with the biodistribution in vivo of directly 99mTc-HMPAO-labelled
CD4+ T cells in response to OVA-pulsed DCs, using SPECT/CT imaging. This
technology has its pitfalls, such as low cellular radiolabelling efficiency, but it
has the
advantage of being dramatically more sensitive than MRI thereby giving insight into
early
migration/accumulation of injected cells in vivo. For instance, a clinical study was
recently
stopped as the engineered melanoma-specific therapeutic T cells that were transferred
into melanoma
patients were cross reactive with an irrelevant antigen in the heart and caused death
due to
infiltration and proliferation in the heart 5. Imaging of the
transferred T cells described above may have changed the outcome of the aforementioned
study. Also,
SPECT/CT can be used to monitor the function of DC vaccines by looking at T-cell migration
and
accumulation of injected T cells following DC vaccination. This is while this technology
is
non-invasive and possesses the ability to image deep in the tissue unlike intra-vital
two-photon and
bioluminescence imaging. In this study, we investigated the in vivo biodistribution
of directly
99mTc-HMPAO-labelled CD4+ T cells in response to OVA-pulsed DCs, as a
model of tumour antigen, using SPECT/CT imaging.
T cells play an important role in protection against tumour invasion and T cells responses
have
been measured ex vivo following injection of tumour-antigen pulsed-DC vaccination
1. In order to determine if the efficacy of anti-cancer therapy can
be assessed at early time points post-DC-vaccination in vivo, primary murine CD4+
T cells were radiolabelled ex vivo, injected and non-invasively imaged by SPET/CT.
CD4+ T cells from DO11.10-Rag−/− mice were isolated and
radiolabelled with 99mTc-HMPAO. The radiolabelling efficiency was between 1.1 and
8.5%. No difference in T-cell viability was observed between radiolabelled and
non-radiolabelled cells (Supporting Information Fig. 1A). To determine the biodistribution
of
99mTc-HMPAO labelled CD4+ T cells in vivo in the absence of antigen,
radiolabelled cells were adoptively transferred i.v. into BALB/c mice and imaged using
NanoSPECT/CT.
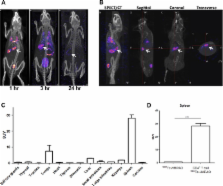
As illustrated (Fig.1A and B and Supporting Information Fig.
1B), 99mTc-HMPAO labelled CD4+ T cells were observed in the spleen 1
hour post-injection. After scanning the mice were culled and organs were dissected
for radioactive
ex vivo biodistribution analysis. The biodistribution data confirmed the presence
of injected
radiolabelled CD4+ T cells within the spleen of recipient mice (standard uptake
value (SUV) = 28.17 ± 4.21) (Fig.1C).
Radiolabelled cells were also present in the lungs 1 hour post-injection (SUV = 7.45
±
5.75). A control group of mice received 99mTc-HMPAO tracer only and showed predominately
clearance to the bladder with significantly less uptake in the spleen (SUV = 0.67
±
0.07, p = 0.001) as compared with the uptake of radiolabelled T cells
(Fig.1D, Supporting Information Fig. 1C and D).
Figure 1
Whole body SPECT/CT imaging of directly 99mTc-HMPAO radiolabelled
CD4+ T cells. A total of 5 × 106 freshly isolated
CD4+ T lymphocytes from DO11.10-Rag−/− mice were directly
radiolabelled with ∼5 MBq of 99mTc-HMPAO and adoptively transferred into BALB/c
recipient mouse. (A) A representative SPECT/CT image of a mouse scanned at 1, 3, and
24 hours after
adoptive transfer (n = 3 mice per time point). (B) SPECT/CT, sagittal,
coronal, and transverse images of a mouse scanned after 1 hour after adoptive transfer.
White arrows
indicate the spleen. (C) After imaging, mice were culled and the biodistribution of
99mTc-HMPAO CD4+ T cells were studied. Data are shown as mean +
SEM of four mice pooled from three individual experiments performed. (D) The standard
uptake value
(SUV) of the spleens of mice receiving either 99mTc-HMPAO or CD4+ T
cells radiolabelled with 99mTc-HMPAO was determined by measuring the presence of
radioactivity in each organs and is shown as mean + SEM of four mice from three individual
experiments. ***p = 0.0001, unpaired two-tailed
t test.
Next, we investigated the migration and accumulation of T cells after DC vaccination.
To achieve
this, BALB/c mice were subcutaneously injected into the right and left lower legs
with DCs pulsed or
not with OVA peptide, respectively. After 24 hours, ∼10 MBq of 99mTc-HMPAO and
CFSE labelled DO11.10-Rag−/− CD4+ T cells were
intravenously injected (Fig.2A). SPECT/CT images were
acquired from 0 to 3 hours post-injection and radiolabelled T cells were observed
in the
experimental right Inguinal LN (i.e., OVA pulsed DCs) but not in the control left
Inguinal LN
(non-pulsed DCs) (Fig.2B and Supporting Information Fig.
2A). To corroborate the in vivo data, the presence of T cells in the right LN was
confirmed by ex
vivo organ biodistribution and flow cytometry (Fig.2C and
Supporting Information Fig. 2B). The analysis of the data demonstrated that OVA antigen-specific
CD4+ T cells have migrated significantly more (p = 0.0008)
to the site of antigen (right Inguinal LN) compared to the control site (left Inguinal
LN). The
specific recruitment of T cells to the OVA-bearing LN was further confirmed by flow
cytometric
analysis (Fig.2D), showing significantly more CFSE labelled
CD4+ T cells in the right inguinal LN (1.01% ± 0.15) compared to the
left inguinal LN (0.31% ± 0.14) (p = 0.0005). Altogether the
results described here suggest that antigen-specific T cells can be radiolabeled with
99mTc-HMPAO and subsequently imaged non-invasively at early time point using NanoSPECT/CT
while remaining viable even with low labelling efficiencies.
Figure 2
SPECT/CT imaging of antigen-specific T-cell response in vivo. (A) BALB/c-derived DCs
were matured
with LPS (1 μg/mL) and pulsed with 2 μg/mL of OVA peptide. The OVA-pulsed DCs were
then subcutaneously injected (1 × 106) into the right heel and unpulsed DCs were
injected (1 × 106) into the left heel. After 24 hours, 5 × 106
99mTc-HMAPO (∼10 MBq) radiolabelled CD4+ T cells isolated from
DO11.10-Rag−/− mice were intravenously injected. (B) After 3 hours the mice
were imaged using NanoSPECT/CT. Radiolabelled CD4+ T cells migrated to the spleen
as well as draining LN as indicated using white arrows (representative of three mice).
(C) Mice were
culled after scan and organs removed for biodistribution. The standard uptake value
(SUV) in the
indicated organs are shown as mean + SEM of four mice pooled from two individual experiments.
***p = 0.0008, unpaired two-tailed t test. (D) Flow
cytometry analysis of cells present in the spleen, right and left inguinal LNs indicating
the
percentage of α-DO11.10 TCR and CFSE-labelled CD4+ T cells after 3 and 96
hours post-injection. Data shown are representative of four mice examined.
Direct ex vivo radiolabelling of leukocytes with radiotracers and gamma imaging is
a routine
clinical procedure within nuclear medicine 6. This has also
previously been achieved in murine models by directly radiolabelling lymphocytes with
111In-oxine 7. Moreover, SPECT/CT was utilised to
image the recruitment of HA-specific 111In-oxine-labelled CTL to HA expressing tumours
8. Also recently, de Vries et al. used 111In-Oxine
labelled DCs pulsed with tumour antigen coupled with [18F]-labelled
3′-flouro-3′-deoxy-thymidine ([18F]FLT) PET imaging to detect
antigen-specific immune responses against DC vaccine in melanoma patients 9. 99mTc
HMPAO has been used routinely for the direct radiolabelling of
white blood cells for clinical imaging 4. However, it is not
informative to compare the imaging of mixed population of cells (such as white blood
cells) with
that of enriched single population of cells. Interestingly, a recent study used 99mTc
HMPAO in imaging of pure eosinphils or neutrophils in humans 10. Here, we used the
SPECT/CT technology to monitor the migration of T cells post-DC
vaccination as a measure of the efficacy of DC vaccination.
We have shown in this study that direct radiolabelling of CD4+ T cells with
99mTc-HMPAO did not induce significant cell death and that the radiolabelled T cells
proliferate in vivo 4 days post-injection that is comparable to what we have previously
reported
2. Although the radiolabelling efficiency was low, it was
sufficient sensitive for visualising adoptively transferred radiolabelled CD4+ T
cells in vivo using SPECT/CT. This was confirmed by organ biodistribution studies.
This
radiolabelling procedure and imaging via SPECT/CT could be a promising method of monitoring
therapeutic intervention in man. Having shown previously that injected antigen-pulsed
DCs migrated
to draining LN 2, we used the same model to study T-cell
activation and migration by adoptively transferred radiolabelled antigen-specific
T cells one day
after DCs vaccination. We demonstrate that migration of T cells to the draining LN
was detected
within 3 hours post-injection of 5 × 106 cells. To our knowledge this is the first
report of non-invasive imaging of early migration (i.e., within 3 hours post-injection)
of
CD4+ T cells to draining LN post-antigen challenge. In contrast, recruitment of
CTLs to tumours in previously published murine models was imaged for example only
after 24 hours
post-injection of 10 × 106 CTL 8. Also, in a
clinical study where DCs were injected intranodally, 111In-labelled DCs were detected
after 3 days and this was correlated with immune activation of T and B cells as these
cells were
activated and proliferated. Using 18F-FLT, the proliferation was detected using PET
imaging. The direct radiolabelling method studied here can be beneficial when tracking
of cells is
required at early time points post-injection. However, direct radiolabelling of mononuclear
cells
using 99mTc-HMPAO has its limitations with sometimes low and variable radiolabelling
efficiencies as well as washout of the radiolabel from the cells 4. This can be overcome
by indirect radiolabelling using a reporter genes, such as Sodium
Iodide Symporter or Herpes Simplex Virus type 1 Thymidine Kinase 11. We have recently
used the Sodium Iodide Symporter reporter gene to study the migration
of murine Treg in mice and are currently correlating their capacity of inducing graft
tolerance to
their migratory property in a model of skin transplantation using SPECT/CT 12. Although
this approach provides many advantages over direct radiolabelling
methods, it requires gene modifications of cells and therefore is not broadly translated
into the
clinic.
Related collections
Most cited references12
- Record: found
- Abstract: found
- Article: not found
Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma.
- Record: found
- Abstract: found
- Article: not found
Guidelines for the labelling of leucocytes with 99mTc-HMPAO
Erik F. J. de Vries, Manel Roca, François Jamar … (2010)
- Record: found
- Abstract: found
- Article: not found