- Record: found
- Abstract: found
- Article: found
Spatiotemporal in vivo tracking of polyclonal human regulatory T cells (Tregs) reveals a role for innate immune cells in Treg transplant recruitment

Read this article at
Abstract
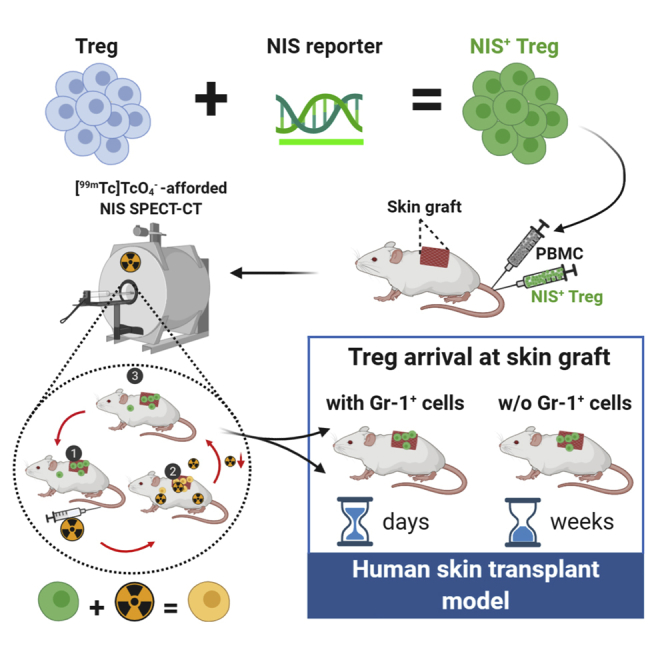
Regulatory T cells (Tregs) are emerging as a new cell-based therapy in solid organ transplantation. Adoptive transfer of Tregs has been shown preclinically to protect from graft rejection, and the safety of Treg therapy has been demonstrated in clinical trials. Despite these successes, the in vivo distribution and persistence of adoptively transferred Tregs remained elusive, which hampers clinical translation. Here we isolated human Tregs using a GMP-compatible protocol and lentivirally transduced them with the human sodium iodide symporter to render them traceable in vivo by radionuclide imaging. Engineered human Tregs were characterized for phenotype, survival, suppressive capacity, and reporter function. To study their trafficking behavior, they were subsequently administered to humanized mice with human skin transplants. Traceable Tregs were quantified in skin grafts by non-invasive nano-single-photon emission computed tomography (nanoSPECT)/computed tomography (CT) for up to 40 days, and the results were validated ex vivo. Using this approach, we demonstrated that Treg trafficking to skin grafts was regulated by the presence of recipient Gr-1 + innate immune cells. We demonstrated the utility of radionuclide reporter gene-afforded quantitative Treg in vivo tracking, addressing a fundamental need in Treg therapy development and offering a clinically compatible methodology for future Treg therapy imaging in humans.
Graphical Abstract
Abstract
Adoptive regulatory T cell (Treg) therapy emerges as a treatment in organ transplantation, but Treg in vivo distribution and persistence remain elusive. Jacob et al. developed a non-invasive long-term Treg therapy tracking method and validated it in humanized models of transplantation. Adaption for future clinical Treg therapy imaging is straightforward.
Related collections
Most cited references65
- Record: found
- Abstract: found
- Article: not found
Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma
- Record: found
- Abstract: found
- Article: not found
Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia
- Record: found
- Abstract: found
- Article: not found