- Record: found
- Abstract: found
- Article: found
Effect of Adenomatous Polyposis Coli Loss on Tumorigenic Potential in Pancreatic Ductal Adenocarcinoma
Read this article at
Abstract
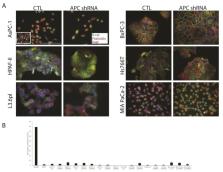
Loss of the Adenomatous Polyposis Coli ( APC) tumor suppressor in colorectal cancer elicits rapid signaling through the Wnt/β-catenin signaling pathway. In contrast to this well-established role of APC, recent studies from our laboratory demonstrated that APC functions through Wnt-independent pathways to mediate in vitro and in vivo models of breast tumorigenesis. Pancreatic ductal adenocarcinoma (PDAC) has an overall median survival of less than one year with a 5-year survival rate of 7.2%. APC is lost in a subset of pancreatic cancers, but the impact on Wnt signaling or tumor development is unclear. Given the lack of effective treatment strategies for pancreatic cancer, it is important to understand the functional implications of APC loss in pancreatic cancer cell lines. Therefore, the goal of this project is to study how APC loss affects Wnt pathway activation and in vitro tumor phenotypes. Using lentiviral shRNA, we successfully knocked down APC expression in six pancreatic cancer cell lines (AsPC-1, BxPC3, L3.6pl, HPAF-II, Hs 766T, MIA PaCa-2). No changes were observed in localization of β-catenin or reporter assays to assess β-catenin/TCF interaction. Despite this lack of Wnt/β-catenin pathway activation, the majority of APC knockdown cell lines exhibit an increase in cell proliferation. Cell migration assays showed that the BxPC-3 and L3.6pl cells were impacted by APC knockdown, showing faster wound healing in scratch wound assays. Interestingly, APC knockdown had no effect on gemcitabine treatment, which is the standard care for pancreatic cancer. It is important to understand the functional implications of APC loss in pancreatic cancer cells lines, which could be used as a target for therapeutics.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer.
- Record: found
- Abstract: found
- Article: not found
Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration.
- Record: found
- Abstract: found
- Article: not found