- Record: found
- Abstract: found
- Article: found
TREX reveals proteins that bind to specific RNA regions in living cells
Read this article at
Abstract
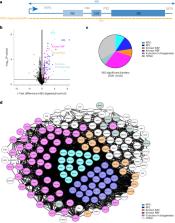
Different regions of RNA molecules can often engage in specific interactions with distinct RNA-binding proteins (RBPs), giving rise to diverse modalities of RNA regulation and function. However, there are currently no methods for unbiased identification of RBPs that interact with specific RNA regions in living cells and under endogenous settings. Here we introduce TREX (targeted RNase H-mediated extraction of crosslinked RBPs)—a highly sensitive approach for identifying proteins that directly bind to specific RNA regions in living cells. We demonstrate that TREX outperforms existing methods in identifying known interactors of U1 snRNA, and reveals endogenous region-specific interactors of NORAD long noncoding RNA. Using TREX, we generated a comprehensive region-by-region interactome for 45S rRNA, uncovering both established and previously unknown interactions that regulate ribosome biogenesis. With its applicability to different cell types, TREX is an RNA-centric tool for unbiased positional mapping of endogenous RNA–protein interactions in living cells.
Abstract
TREX introduces an RNA-centric tool for identifying proteins binding to specific RNA regions in living cells.
Related collections
Most cited references82
- Record: found
- Abstract: found
- Article: not found
STAR: ultrafast universal RNA-seq aligner.
- Record: found
- Abstract: found
- Article: not found
KEGG: kyoto encyclopedia of genes and genomes.

- Record: found
- Abstract: found
- Article: found