- Record: found
- Abstract: found
- Article: found
The Scavenger Function of Liver Sinusoidal Endothelial Cells in Health and Disease
Read this article at
Abstract
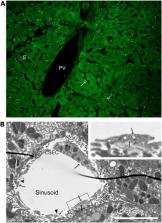
The aim of this review is to give an outline of the blood clearance function of the liver sinusoidal endothelial cells (LSECs) in health and disease. Lining the hundreds of millions of hepatic sinusoids in the human liver the LSECs are perfectly located to survey the constituents of the blood. These cells are equipped with high-affinity receptors and an intracellular vesicle transport apparatus, enabling a remarkably efficient machinery for removal of large molecules and nanoparticles from the blood, thus contributing importantly to maintain blood and tissue homeostasis. We describe here central aspects of LSEC signature receptors that enable the cells to recognize and internalize blood-borne waste macromolecules at great speed and high capacity. Notably, this blood clearance system is a silent process, in the sense that it usually neither requires or elicits cell activation or immune responses. Most of our knowledge about LSECs arises from studies in animals, of which mouse and rat make up the great majority, and some species differences relevant for extrapolating from animal models to human are discussed. In the last part of the review, we discuss comparative aspects of the LSEC scavenger functions and specialized scavenger endothelial cells (SECs) in other vascular beds and in different vertebrate classes. In conclusion, the activity of LSECs and other SECs prevent exposure of a great number of waste products to the immune system, and molecules with noxious biological activities are effectively “silenced” by the rapid clearance in LSECs. An undesired consequence of this avid scavenging system is unwanted uptake of nanomedicines and biologics in the cells. As the development of this new generation of therapeutics evolves, there will be a sharp increase in the need to understand the clearance function of LSECs in health and disease. There is still a significant knowledge gap in how the LSEC clearance function is affected in liver disease.
Related collections
Most cited references251
- Record: found
- Abstract: found
- Article: not found
Resolving the fibrotic niche of human liver cirrhosis at single cell level

- Record: found
- Abstract: found
- Article: found