- Record: found
- Abstract: found
- Article: found
Long non-coding RNA LINC00520 promotes the proliferation and metastasis of malignant melanoma by inducing the miR-125b-5p/EIF5A2 axis
Read this article at
Abstract
Background
Long intergenic non-protein coding RNA 520 (LINC00520), a novel identified lncRNA, has been shown to modulate the malignant phenotype of tumor cells in some malignant tumors. However, the exact role and molecular mechanism of LINC00520 in malignant melanoma has not been studied.
Methods
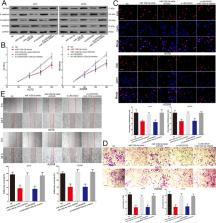
The expression of LINC00520 in melanoma tissues were detected by using RNA-seq analysis and qRT-PCR. Melanoma cases from the public databases (The Cancer Genome Atlas (TCGA), GEO#GSE15605, GEO#GSE34460 and GEO#GSE24996) were included in this study. CCK-8 assay, EdU assay, transwell and scratch wound assay were used to explore the role of LINC00520 in melanoma cells. Luciferase reporter assays, MS2-RIP, RNA pull-down and RNA-ChIP assay were used to demonstrate the molecular biological mechanism of LINC00520 in melanoma.
Results
We found that LICN00520 was found to be overexpressed in melanoma tissue. High expression of LICN00520 is a risk factor for the prognosis of melanoma patients. LINC00520 promotes the proliferation, invasion and migration of melanoma cells. LICN00520 exerted its oncogenic role by competitive binding miR-125b-5p to promote Eukaryotic initiation factor 5A2 (EIF5A2) expression. We also showed that LICN00520 promotes the growth and metastasis of melanoma in vivo through regulating miR-125b-5p/EIF5A2 axis.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: found
Tumor-associated stromal cells as key contributors to the tumor microenvironment

- Record: found
- Abstract: found
- Article: found
Long noncoding RNA LINC00511 contributes to breast cancer tumourigenesis and stemness by inducing the miR-185-3p/E2F1/Nanog axis
- Record: found
- Abstract: found
- Article: not found