- Record: found
- Abstract: found
- Article: found
Klotho-mediated activation of the anti-oxidant Nrf2/ARE signal pathway affects cell apoptosis, senescence and mobility in hypoxic human trophoblasts: involvement of Klotho in the pathogenesis of preeclampsia
Read this article at
Abstract
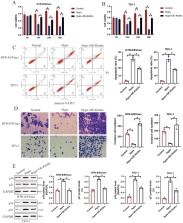
The anti-aging gene Klotho is implicated in the pathogenesis of preeclampsia (PE), which is a pregnancy disease characterized by hypertension and proteinuria. Oxidative stress is closely associated with the worse outcomes in PE, and Klotho can eliminate Reactive Oxygen Species (ROS), but it is still unclear whether Klotho regulates PE pathogenesis through modulating oxidative damages. Here, by analyzing the clinical data, we found that Klotho was aberrantly downregulated in PE umbilical cord serum and placental tissues, compared to their normal counterparts. In in vitro experiments, the human trophoblasts were subjected to hypoxic pressure to establish the PE models, and we confirmed that hypoxia also decreased the expression levels of Klotho in those trophoblasts. In addition, through performing functional experiments, we confirmed that hypoxia promoted oxidative damages, cell apoptosis and senescence, whereas suppressed cell invasion in human trophoblasts, which were all reversed overexpressing Klotho. The following mechanical experiments verified that Klotho increased the levels of nuclear Nrf2, total Nrf2, SOD2 and NQO1 to activate the anti-oxidant Nrf2/ARE signal pathway, and silencing of Nrf2 abrogated the protective effects of Klotho overexpression on hypoxic human trophoblasts. Consistently, in in vivo experiments, Klotho overexpression restrained oxidative damages and facilitated cell mitosis in PE rats’ placental tissues. In conclusion, this study validated that Klotho activated the Nrf2/ARE signal pathway to eliminate hypoxia-induced oxidative damages, cell apoptosis and senescence to recover normal cellular functions in human trophoblasts, and our data supported that Klotho could be used as novel biomarker for PE diagnosis and treatment.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases.
- Record: found
- Abstract: found
- Article: not found
