- Record: found
- Abstract: found
- Article: not found
Illuminating Biological Interactions with in Vivo Protein Footprinting
Read this article at
Abstract

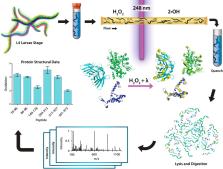
Protein footprinting coupled with mass spectrometry is being increasingly used for the study of protein interactions and conformations. The hydroxyl radical footprinting method, fast photochemical oxidation of proteins (FPOP), utilizes hydroxyl radicals to oxidatively modify solvent accessible amino acids. Here, we describe the further development of FPOP for protein structural analysis in vivo (IV-FPOP) with Caenorhabditis elegans. C. elegans, part of the nematode family, are used as model systems for many human diseases. The ability to perform structural studies in these worms would provide insight into the role of structure in disease pathogenesis. Many parameters were optimized for labeling within the worms including the microfluidic flow system and hydrogen peroxide concentration. IV-FPOP was able to modify several hundred proteins in various organs within the worms. The method successfully probed solvent accessibility similarily to in vitro FPOP, demonstrating its potential for use as a structural technique in a multiorgan system. The coupling of the method with mass spectrometry allows for amino-acid-residue-level structural information, a higher resolution than currently available in vivo methods.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
The fluorescent toolbox for assessing protein location and function.
- Record: found
- Abstract: found
- Article: not found
Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics.
- Record: found
- Abstract: not found
- Article: not found
