- Record: found
- Abstract: found
- Article: found
Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis
Read this article at
Abstract
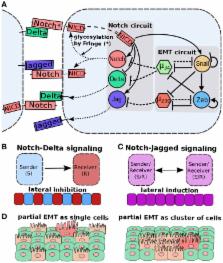
Transitions between epithelial and mesenchymal phenotypes – the epithelial to mesenchymal transition (EMT) and its reverse the mesenchymal to epithelial transition (MET) – are hallmarks of cancer metastasis. While transitioning between the epithelial and mesenchymal phenotypes, cells can also attain a hybrid epithelial/mesenchymal (E/M) (i.e., partial or intermediate EMT) phenotype. Cells in this phenotype have mixed epithelial (e.g., adhesion) and mesenchymal (e.g., migration) properties, thereby allowing them to move collectively as clusters. If these clusters reach the bloodstream intact, they can give rise to clusters of circulating tumor cells (CTCs), as have often been seen experimentally. Here, we review the operating principles of the core regulatory network for EMT/MET that acts as a “three-way” switch giving rise to three distinct phenotypes – E, M and hybrid E/M – and present a theoretical framework that can elucidate the role of many other players in regulating epithelial plasticity. Furthermore, we highlight recent studies on partial EMT and its association with drug resistance and tumor-initiating potential; and discuss how cell–cell communication between cells in a partial EMT phenotype can enable the formation of clusters of CTCs. These clusters can be more apoptosis-resistant and have more tumor-initiating potential than singly moving CTCs with a wholly mesenchymal (complete EMT) phenotype. Also, more such clusters can be formed under inflammatory conditions that are often generated by various therapies. Finally, we discuss the multiple advantages that the partial EMT or hybrid E/M phenotype have as compared to a complete EMT phenotype and argue that these collectively migrating cells are the primary “bad actors” of metastasis.
Related collections
Most cited references144
- Record: found
- Abstract: found
- Article: not found
Identification of pancreatic cancer stem cells.

- Record: found
- Abstract: found
- Article: found
Plasticity of cell migration: a multiscale tuning model
- Record: found
- Abstract: found
- Article: not found
Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.