- Record: found
- Abstract: found
- Article: found
The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges
Read this article at
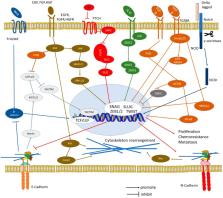
Abstract
Epithelial-to-Mesenchymal Transition (EMT) has been shown to be crucial in tumorigenesis where the EMT program enhances metastasis, chemoresistance and tumor stemness. Due to its emerging role as a pivotal driver of tumorigenesis, targeting EMT is of great therapeutic interest in counteracting metastasis and chemoresistance in cancer patients. The hallmark of EMT is the upregulation of N-cadherin followed by the downregulation of E-cadherin, and this process is regulated by a complex network of signaling pathways and transcription factors. In this review, we summarized the recent understanding of the roles of E- and N-cadherins in cancer invasion and metastasis as well as the crosstalk with other signaling pathways involved in EMT. We also highlighted a few natural compounds with potential anti-EMT property and outlined the future directions in the development of novel intervention in human cancer treatments. We have reviewed 287 published papers related to this topic and identified some of the challenges faced in translating the discovery work from bench to bedside.
Related collections
Most cited references231
- Record: found
- Abstract: found
- Article: not found
Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy.
- Record: found
- Abstract: found
- Article: not found
High absorption but very low bioavailability of oral resveratrol in humans.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.